Explore the broad array of multiple myeloma clinical trials
DARZALEX® in newly diagnosed multiple myeloma


- Patients in the DARZALEX® + VMP arm received DARZALEX® 16 mg/kg until disease progression or unacceptable toxicity1
- Participants received bortezomib 1.3 mg/m2 as subcutaneous injection, twice weekly at Weeks 1, 2, 4, and 5 (Cycle 1) followed by once weekly at Weeks 1, 2, 4, and 5 (Cycles 2-9); melphalan 9 mg/m2 and prednisone 60 mg/m2 were orally administered on Days 1 to 4 of the nine 6-week cycles (Cycles 1-9). Per protocol, VMP treatment was discontinued in both arms after 9 cycles1*
- Primary endpoint was efficacy evaluated by progression-free survival (PFS) based on International Myeloma Working Group (IMWG) criteria2
- Key secondary endpoints included overall response rate (ORR), rates of very good partial response (VGPR) or better, complete response (CR) or better, negative status for minimal residual disease (MRD), and overall survival (OS)2
- Additional endpoints included time to response, duration of response, safety, and side effect profile2




with VMP alone1




- In responders, median time to response was 0.79 months (range: 0.4-15.5 months) in the DARZALEX® + VMP group and 0.82 months (range: 0.7-12.6 months) in the VMP group1
- 42.6% of patients achieved CR or better with DARZALEX® + VMP vs 24.4% with VMP alone1
- Median duration of response had not yet been reached with DARZALEX® + VMP vs 21.3 months (range: 0.5+, 23.7+) with VMP alone1

(n=78)
(95% CI: 18.0, 27.0)

(n=22)
(95% CI: 3.9, 9.2)
- Minimal residual disease (MRD) based on threshold of 10-5 using a next-generation sequencing assay
- In patients with CR or better, the MRD negativity rate was 49.7% (n=74) (95% CI: 41.4, 58.0) in the DARZALEX® + VMP arm vs 25.3% (n=22) (95% CI: 16.6, 35.7) with VMP arm


- The most frequent adverse reactions (≥20% with at least 5% greater frequency in the DVMP arm) were infusion reactions, upper respiratory tract infection, and peripheral edema1
- Serious adverse reactions with at least a 2% greater incidence in the DARZALEX® + VMP arm compared with the VMP arm were pneumonia (DVMP 11% vs VMP 4%), upper respiratory tract infection (DVMP 5% vs VMP 1%), and pulmonary edema (DVMP 2% vs VMP 0%)1


- Grade 3 or 4 infections were 23% with DARZALEX® + VMP vs 15% with VMP alone2


- For 37% of patients, IRRs (any grade) occurred with the Week 1 (16 mg/kg) infusion; for 2% of patients, with the Week 2 infusion; and cumulatively, for 6% of patients with subsequent infusions
- Median time to onset of an IRR was 1.5 hours (range: 0–73 hours)
- Incidence of infusion modification due to reactions was 36%
- DARZALEX® can cause severe IRRs. Severe IRRs included bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension, and blurred vision
- Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with DARZALEX® infusion. If ocular symptoms occur, interrupt DARZALEX® infusion and seek immediate ophthalmologic evaluation prior to restarting DARZALEX®


- Type and screen patients before starting DARZALEX®
- Inform blood banks when a patient is on DARZALEX®
- Identify any DARZALEX®-treated blood samples
- Ask patients to tell other healthcare professionals that they've taken DARZALEX®


- In an ongoing part 2 of the study, patients underwent a second randomization in both treatment groups to either observation or DARZALEX® Q8W monotherapy for maintenance. The efficacy and safety of this maintenance regimen is currently being evaluated3
- DARZALEX® dosing and administration: IV, 16 mg/kg actual body weight QW Cycles 1–2 and Q2W Cycles 3–4 (induction phase); Q2W Cycles 5–6 (consolidation phase)1
- VTd dosing and administration: Bortezomib (V) SC or IV, 1.3 mg/m2 BSA twice weekly for 2 weeks on Days 1, 4, 8, and 11 of repeated 28-day (4-week) induction cycles (Cycles 1–4) and 2 consolidation cycles (Cycles 5–6); thalidomide (T) PO, 100 mg daily during the 6 bortezomib cycles; dexamethasone (d) PO or IV, 40 mg on Days 1, 2, 8, 9, 15, 16, 22, and 23 of Cycles 1 and 2, and at 40 mg on Days 1–2 and 20 mg on subsequent dosing days (Days 8, 9, 15, 16) of Cycles 3–4. Dexamethasone 20 mg was administered on Days 1, 2, 8, 9, 15, and 16 in Cycles 5–6. On the days of DARZALEX® infusion, the dexamethasone dose was administered intravenously as a pre-infusion medication1
- Primary endpoint was stringent complete response (sCR) rate at Day 100 post-transplant3
- Key secondary endpoints included: rate of CR or better, PFS from first randomization and OS from first randomization3
- Patient characteristics: the median age was 58 years; 59% male; 48% had an ECOG performance score of 0, 42% had an ECOG performance score of 1, and 10% had an ECOG performance score of 2; 40% had ISS Stage I, 45% had ISS Stage II, and 15% had ISS Stage III disease1
- Approval of frontline treatment of transplant-eligible patients with multiple myeloma is based on the response evaluation of Part 1 only of this trial at Day 100 post-transplant





- Response rates for DARZALEX® + VTd vs VTd alone were, respectively: sCR, 28.9% vs 20.3%; CR, 9.9% vs 5.7%; VGPR, 44.6% vs 52.0%; PR, 9.2% vs 11.8%


- 489 (90%) with DARZALEX® + VTd and 484 (89%) with VTd alone3
- 85% of patients in the DARZALEX® + VTd group and 81% of those in the VTd group completed all 4 induction and both consolidation cycles at 18.8 months (median follow-up)1,3




- Discontinuation rates due to any adverse event: 7% with DVTd vs 8% with VTd3
- Infusion reactions with DVTd occurred in 35% of patients; 3% were Grade 3 and <1% were Grade 41
- Most infusion reactions occurred during the first infusion3



- Administer pre-infusion and post-infusion medications to reduce the risk of infusion reactions [see section 2.3 of the DARZALEX® full Prescribing Information]1
- For 37% of patients, IRRs (any grade) occurred with the Week 1 (16 mg/kg) infusion; for 2% of patients, with the Week 2 infusion; and cumulatively, for 6% of patients with subsequent infusions
- Median time to onset of an IRR was 1.5 hours (range: 0–73 hours)
- Incidence of infusion modification due to reactions was 36%
- DARZALEX® can cause severe IRRs. Severe IRRs included bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension, and blurred vision
- Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with DARZALEX® infusion. If ocular symptoms occur, interrupt DARZALEX® infusion and seek immediate ophthalmologic evaluation prior to restarting DARZALEX®


- Type and screen patients before starting DARZALEX®
- Inform blood banks when a patient is on DARZALEX®
- Identify any DARZALEX®-treated blood samples
- Ask patients to tell other healthcare professionals that they've taken DARZALEX®
DARZALEX® in relapsed or refractory multiple myeloma
- A majority (86%) of patients received prior treatment with a proteasome inhibitor (PI)1
- More than half (55%) of patients received prior treatment with an immunomodulatory agent1
Patients were refractory to prior therapies1*
- 27% to the last line of treatment
- 18% to a PI only
- 21% to bortezomib
DRd=DARZALEX® (D) + lenalidomide (R) + dexamethasone (d); IV=intravenous; PD=progressive disease; PO=by mouth; QW=once weekly; Q2W=every 2 weeks; Q4W=every 4 weeks; Rd=lenalidomide (R) + dexamethasone (d).
*Please note that this is not a full list of prior therapies reported at baseline and that lenalidomide-refractory patients were excluded from the study.4
ECOG=Eastern Cooperative Oncology Group; Rd=lenalidomide (R) + dexamethasone (d).
was 13.5 months1,4
†Efficacy was evaluated by PFS based on International Myeloma Working Group (IMWG) criteria.1
National Comprehensive Cancer Network® (NCCN®) Category 1, preferred
Daratumumab* (D) in combination with lenalidomide (R) and dexamethasone (d) is recommended by the NCCN Guidelines as a Category 1 preferred† therapeutic option for patients with relapsed or refractory multiple myeloma‡
*Daratumumab includes both daratumumab and hyaluronidase-fihj (DARZALEX FASPRO®) for subcutaneous injection and daratumumab (DARZALEX®) for intravenous infusion. Daratumumab and hyaluronidase-fihj for subcutaneous injection has different dosing and administration instructions compared to daratumumab for intravenous infusion.
†See nccn.org for definitions of NCCN Categories of Preference and NCCN Categories of Evidence and Consensus.
‡Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V3.2023. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed December 12, 2022. To view the most recent and complete version of the guideline, go online to www.nccn.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
CI=confidence interval; DRd=DARZALEX® (D) + lenalidomide (R) + dexamethasone (d); HR=hazard ratio; mPFS=median progression-free survival; PFS=progression-free survival; Rd=lenalidomide (R) + dexamethasone (d).
Complete response rate more than doubled with DARZALEX® + Rd1
Almost all (91%) patients responded to DARZALEX® + Rd.
CR=complete response; ORR=overall response rate; PR=partial response; Rd=lenalidomide (R) + dexamethasone (d); sCR=stringent complete response; VGPR=very good partial response.
Deeper and sustained responses were demonstrated with DARZALEX® + Rd vs Rd1
With DARZALEX® + Rd, median time to first response was 1 month (range: 0.9 to 13 months).
Rd=lenalidomide (R) + dexamethasone (d).
aUpper respiratory tract infection, bronchitis, sinusitis, respiratory tract infection viral, rhinitis, pharyngitis, respiratory tract infection, metapneumovirus infection, tracheobronchitis, viral upper respiratory tract infection, laryngitis, respiratory syncytial virus infection, staphylococcal pharyngitis, tonsillitis, viral pharyngitis, acute sinusitis, nasopharyngitis, bronchiolitis, bronchitis viral, pharyngitis streptococcal, tracheitis, upper respiratory tract infection bacterial, bronchitis bacterial, epiglottitis, laryngitis viral, oropharyngeal candidiasis, respiratory moniliasis, viral rhinitis, acute tonsillitis, rhinovirus infection.
bInfusion-related reaction includes terms determined by investigators to be related to infusion.
cCough, productive cough, allergic cough.
dDyspnea, dyspnea exertional.
Note: Adverse reactions that occurred in >10% of patients and with at least 5% greater frequency in the DARZALEX® + Rd arm are listed.
- Infusion reaction includes terms determined by investigators to be related to infusion. Severe infusion reactions included bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension, and blurred vision1
- The most frequent adverse reactions (≥20%) with DARZALEX® + Rd (DRd) were infusion reactions, diarrhea, nausea, fatigue, pyrexia, upper respiratory tract infection, muscle spasms, cough, and dyspnea1
- Serious adverse reactions with at least a 2% greater incidence in the DARZALEX® + Rd arm compared to the Rd arm were pneumonia (DRd 12% vs Rd 10%), upper respiratory tract infection (DRd 7% vs Rd 4%), influenza and pyrexia (DRd 3% vs Rd 1% for each)1
Rd=lenalidomide (R) + dexamethasone (d).
- Grade 3/4 infections between study arms: 28% vs 23% with DARZALEX® + Rd and Rd, respectively4
- Discontinuation rates due to adverse reactions with DARZALEX® + Rd were similar to Rd alone (7% vs 8%, respectively)1
37%
of patients had IRRs with the Week 1 infusion
2%
of patients had IRRs with the Week 2 infusion
6%
of patients had IRRs with subsequent infusions
IRRs=infusion-related reactions.
- For 37% of patients, IRRs (any grade) occurred with the Week 1 (16 mg/kg) infusion; for 2% of patients, with the Week 2 infusion; and cumulatively, for 6% of patients with subsequent infusions
- Median time to onset of an IRR was 1.5 hours (range: 0–73 hours)
- Incidence of infusion modification due to reactions was 36%
- DARZALEX® can cause severe IRRs. Severe IRRs included bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension, and blurred vision
- Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with DARZALEX® infusion. If ocular symptoms occur, interrupt DARZALEX® infusion and seek immediate ophthalmologic evaluation prior to restarting DARZALEX®
For IRRs of any grade/severity, immediately interrupt the DARZALEX® infusion and manage symptoms. Management of IRRs may further require reduction in the rate of infusion or treatment discontinuation of DARZALEX® as outlined below.1
IRR=infusion-related reaction.
DARZALEX® binds to CD38 found on red blood cells (RBCs) and results in a positive indirect antiglobulin test (indirect Coombs test) that may persist for up to 6 months after the last DARZALEX® infusion.
Reminders
- Type and screen patients before starting DARZALEX®
- Inform blood banks when a patient is on DARZALEX®
- Identify any DARZALEX®-treated blood samples
- Ask patients to tell other healthcare professionals that they've taken DARZALEX®
Brochure on daratumumab and serological testing
Identification card to give to patients taking daratumumab
DVd=DARZALEX® (D) + bortezomib (V) + dexamethasone (d); ECOG=Eastern Cooperative Oncology Group; Vd=bortezomib (V) + dexamethasone (d).
- A majority (76%) of patients received prior treatment with an immunomodulatory agent1
- More than half (69%) of patients received prior treatment with a proteasome inhibitor (PI)1
Patients were refractory to prior therapies1*
- 32% to the last line of treatment
- 33% to an immunomodulatory agent only
- 24% in the DARZALEX® + Vd arm and 33% in the Vd arm to lenalidomide
Vd=bortezomib (V) + dexamethasone (d).
*Please note that this is not a full list of prior therapies reported at baseline and that bortezomib-refractory patients were excluded from the study.
ECOG=Eastern Cooperative Oncology Group; Vd=bortezomib (V) + dexamethasone (d).
was 7.4 months1,5
†Efficacy was evaluated by PFS based on International Myeloma Working Group (IMWG) criteria.1
National Comprehensive Cancer Network® (NCCN®) Category 1, preferred
Daratumumab* (D) in combination with bortezomib (V) and dexamethasone (d) is recommended by the NCCN Guidelines as a Category 1 preferred† therapeutic option for patients with relapsed or refractory multiple myeloma‡
*Daratumumab includes both daratumumab and hyaluronidase-fihj (DARZALEX FASPRO®) for subcutaneous injection and daratumumab (DARZALEX®) for intravenous infusion. Daratumumab and hyaluronidase-fihj for subcutaneous injection has different dosing and administration instructions compared to daratumumab for intravenous infusion.
†See nccn.org for definitions of NCCN Categories of Preference and NCCN Categories of Evidence and Consensus.
‡Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V3.2023. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed December 12, 2022. To view the most recent and complete version of the guideline, go online to www.nccn.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
CI=confidence interval; DVd=DARZALEX® (D) + bortezomib (V) + dexamethasone (d); HR=hazard ratio; PFS=progression-free survival; Vd=bortezomib (V) + dexamethasone (d).
Complete response rate more than doubled with DARZALEX® + Vd1
The majority (79%) of patients responded to DARZALEX® + Vd.
CR=complete response; ORR=overall response rate; PR=partial response; sCR=stringent complete response; Vd=bortezomib (V) + dexamethasone (d); VGPR=very good partial response.
Deeper and sustained responses were demonstrated with DARZALEX® + Vd vs Vd1
With DARZALEX® + Vd, median time to first response was 0.8 months (range: 0.7 to 4 months).
Vd=bortezomib (V) + dexamethasone (d).
aInfusion-related reaction includes terms determined by investigators to be related to infusion.
bUpper respiratory tract infection, bronchitis, sinusitis, respiratory tract infection viral, rhinitis, pharyngitis, respiratory tract infection, metapneumovirus infection, tracheobronchitis, viral upper respiratory tract infection, laryngitis, respiratory syncytial virus infection, staphylococcal pharyngitis, tonsillitis, viral pharyngitis, acute sinusitis, nasopharyngitis, bronchiolitis, bronchitis viral, pharyngitis streptococcal, tracheitis, upper respiratory tract infection bacterial, bronchitis bacterial, epiglottitis, laryngitis viral, oropharyngeal candidiasis, respiratory moniliasis, viral rhinitis, acute tonsillitis, rhinovirus infection.
cCough, productive cough, allergic cough.
dEdema peripheral, edema, generalized edema, peripheral swelling.
eDyspnea, dyspnea exertional.
Note: Adverse reactions that occurred in >10% of patients and with at least 5% greater frequency in the DARZALEX® + Vd arm are listed.
- Infusion reaction includes terms determined by investigators to be related to infusion. Severe infusion reactions included bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension, and blurred vision1
- The most frequent adverse reactions (>20%) with DARZALEX® + Vd (DVd) were infusion reactions, diarrhea, peripheral edema, upper respiratory tract infection, peripheral sensory neuropathy, cough, and dyspnea1
- Serious adverse reactions with at least a 2% greater incidence in the DVd arm compared to the Vd arm were upper respiratory tract infection (DVd 5% vs Vd 2%), diarrhea, and atrial fibrillation (DVd 2% vs Vd 0% for each)1
Vd=bortezomib (V) + dexamethasone (d).
- Grade 3/4 infections were similar between study arms: 21% vs 19% with DARZALEX® + Vd and Vd, respectively5
- Discontinuation rates due to adverse reactions with DARZALEX® + Vd were similar to Vd alone (7% vs 9%, respectively)1
37%
of patients had IRRs with the Week 1 infusion
2%
of patients had IRRs with the Week 2 infusion
6%
of patients had IRRs with subsequent infusions
IRRs=infusion-related reactions.
- For 37% of patients, IRRs (any grade) occurred with the Week 1 (16 mg/kg) infusion; for 2% of patients, with the Week 2 infusion; and cumulatively, for 6% of patients with subsequent infusions
- Median time to onset of an IRR was 1.5 hours (range: 0–73 hours)
- Incidence of infusion modification due to reactions was 36%
- DARZALEX® can cause severe IRRs. Severe IRRs included bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension, and blurred vision
- Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with DARZALEX® infusion. If ocular symptoms occur, interrupt DARZALEX® infusion and seek immediate ophthalmologic evaluation prior to restarting DARZALEX®
For IRRs of any grade/severity, immediately interrupt the DARZALEX® infusion and manage symptoms. Management of IRRs may further require reduction in the rate of infusion or treatment discontinuation of DARZALEX® as outlined below.1
IRR=infusion-related reaction.
DARZALEX® binds to CD38 found on red blood cells (RBCs) and results in a positive indirect antiglobulin test (indirect Coombs test) that may persist for up to 6 months after the last DARZALEX® infusion.
Reminders
- Type and screen patients before starting DARZALEX®
- Inform blood banks when a patient is on DARZALEX®
- Identify any DARZALEX®-treated blood samples
- Ask patients to tell other healthcare professionals that they've taken DARZALEX®
Brochure on daratumumab and serological testing
Identification card to give to patients taking daratumumab
ANC=absolute neutrophil count; BIW=twice weekly; DKd=DARZALEX® (D) + carfilzomib (K) + dexamethasone (d); IV=intravenous; Kd=carfilzomib (K) + dexamethasone (d); PD=progressive disease; QW=weekly; Q2W=every 2 weeks; Q4W=every 4 weeks.
*For patients aged >75 years on a reduced dexamethasone dose of 20 mg, the entire 20 mg dose was given as pre-medication on days when DARZALEX® was administered.1
- The median age was 64 years (range: 29–84 years); 58% male; 79% White, 14% Asian, and 2% Black
- Patients had received a median of 2 prior lines of therapy and 58% of patients had received prior autologous stem cell transplantation (ASCT)
- 92% received a prior proteasome inhibitor (PI), and of those, 34% were refractory to a PI-including regimen
- 42% of patients had received prior lenalidomide, and of those, 33% were refractory to a lenalidomide-containing regimen
EQUULEUS: an open-label, multicohort study of once-weekly DARZALEX® + Kd1
EQUULEUS evaluated the efficacy and safety of DARZALEX® in combination with once-weekly carfilzomib (70 mg/m2) and dexamethasone in patients with relapsed or refractory multiple myeloma who had received 1 to 3 prior lines of therapy.
Baseline patient characteristics (n=85)1

of patients had received prior lenalidomide

of patients were refractory to lenalidomide

of patients were refractory to both a PI and an immunomodulatory agent
PI=proteasome inhibitor.
National Comprehensive Cancer Network® (NCCN®) Category 1, preferred
Daratumumab* (D) in combination with carfilzomib (K) and dexamethasone (d) is recommended by the NCCN Guidelines as a Category 1 preferred† therapeutic option for patients with relapsed or refractory multiple myeloma‡
*Daratumumab includes both daratumumab and hyaluronidase-fihj (DARZALEX FASPRO®) for subcutaneous injection and daratumumab (DARZALEX®) for intravenous infusion. Daratumumab and hyaluronidase-fihj for subcutaneous injection has different dosing and administration instructions compared to daratumumab for intravenous infusion.
†See nccn.org for definitions of NCCN Categories of Preference and NCCN Categories of Evidence and Consensus.
‡Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V3.2023. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed December 12, 2022. To view the most recent and complete version of the guideline, go online to www.nccn.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
CI=confidence interval; DKd=DARZALEX® (D) + carfilzomib (K) + dexamethasone (d); HR=hazard ratio; Kd=carfilzomib (K) + dexamethasone (d); PFS=progression-free survival.
PFS was assessed by an independent review committee (IRC) using International Myeloma Working Group (IMWG) response criteria.1
CI=confidence interval; CR=complete response; DKd=DARZALEX® (D) + carfilzomib (K) + dexamethasone (d); Kd=carfilzomib (K) + dexamethasone (d); ORR=overall response rate; PR=partial response; VGPR=very good partial response.
PFS was assessed by an independent review committee (IRC) using International Myeloma Working Group (IMWG) response criteria.1
*DKd (95% CI: 80, 88).
†Kd (95% CI: 67, 81).
Superior minimal residual disease (MRD) negativity‡ with DARZALEX® + Kd vs Kd alone1
CI=confidence interval; CR=complete response; DKd=DARZALEX® (D) + carfilzomib (K) + dexamethasone (d); Kd=carfilzomib (K) + dexamethasone (d); MRD=minimal residual disease.
‡Based on a sensitivity threshold of 10-5 using a next-generation sequencing assay (clonoSEQ®). MRD negativity was defined according to the 2016 International Myeloma Working Group Uniform Response Criteria (IMWG-URC).
§DKd (95% CI: 9, 17).
∥Kd (95% CI: 0.2, 4.6).
¶P value from the stratified Cochran Mantel-Haenszel Chi-Square test.
EQUULEUS: demonstrated response with once weekly DARZALEX® + Kd1
CI=confidence interval; CR=complete response; Kd=carfilzomib (K) + dexamethasone (d); PR=partial response; sCR=stringent complete response; VGPR=very good partial response.
CANDOR: DARZALEX® + Kd safety profile
Most frequent adverse reactions reported in ≥15% of patients who received DARZALEX® + Kd1
DKd=DARZALEX® (D) + carfilzomib (K) + dexamethasone (d); Kd=carfilzomib (K) + dexamethasone (d).
aThe incidence of infusion-related reactions is based on a group of symptoms (including hypertension, pyrexia, rash, myalgia, hypotension, blood pressure increased, urticaria, acute kidney injury, bronchospasm, face edema, hypersensitivity, rash, syncope, wheezing, eye pruritus, eyelid edema, renal failure, swelling face) related to infusion reactions, which occurred within 1 day after DKd or Kd administration.
bRespiratory tract infection includes respiratory tract infection, lower respiratory tract infection, upper respiratory tract infection, and viral upper respiratory tract infection.
cThrombocytopenia includes platelet count decreased and thrombocytopenia.
dAnemia includes anemia, hematocrit decreased, and hemoglobin decreased.
eFatigue includes fatigue and asthenia.
fAnemia includes anemia, hematocrit decreased, and hemoglobin decreased.
gIncludes fatal adverse reactions.
- Median treatment duration of 16.1 months (range: 0.1–23.7) with DKd and 9.3 months (range: 0.1–22.4 months) in Kd1
- Serious adverse reactions occurred in 56% of patients who received DARZALEX® in combination with Kd and 46% of patients who received Kd alone1
- The most frequent serious adverse reactions reported in the DKd arm as compared with the Kd arm were pneumonia (DKd 14% vs Kd 9%), pyrexia (DKd 4.2% vs Kd 2.0%), influenza (DKd 3.9% vs Kd 1.3%), sepsis (DKd 3.9% vs Kd 1.3%), anemia (DKd 2.3% vs Kd 0.7%), bronchitis (DKd 1.9% vs Kd 0%), and diarrhea (DKd 1.6% vs Kd 0%)1
- Permanent discontinuation of DARZALEX® due to an adverse reaction occurred in 9% of patients1
- Fatal adverse reactions within 30 days of the last dose of any study treatment occurred in 10% of 308 patients who received DARZALEX® in combination with Kd vs 5% of 153 patients who received Kd, and the most frequent fatal adverse reaction was infection (4.5% vs 2.6%)1
EQUULEUS: DARZALEX® + Kd safety profile
Most frequent adverse reactions reported in ≥15% of patients receiving DARZALEX® + once weekly carfilzomib and dexamethasone1
DKd=DARZALEX® (D) + carfilzomib (K) + dexamethasone (d); Kd=carfilzomib (K) + dexamethasone (d).
aThrombocytopenia includes platelet count decreased and thrombocytopenia.
bFatigue includes fatigue and asthenia.
cThe incidence of infusion-related reactions is based on a group of symptoms (including hypertension, pyrexia, rash, myalgia, hypotension, blood pressure increased, urticaria, acute kidney injury, bronchospasm, face edema, hypersensitivity, rash, syncope, wheezing, eye pruritus, eyelid edema, renal failure, swelling face) related to infusion reactions, which occurred within 1 day after DKd administration.
dRespiratory tract infection includes respiratory tract infection, lower respiratory tract infection, upper respiratory tract infection, and viral upper respiratory tract infection.
eAnemia includes anemia, hematocrit decreased, and hemoglobin decreased.
fCough includes productive cough and cough.
gNeutropenia includes neutrophil count decreased and neutropenia.
hLymphopenia includes lymphocyte count decreased and lymphopenia.
- Median treatment duration of 19.8 months (range: 0.3–34.5 months)1
- Serious adverse reactions were reported in 48% of patients1
- The most frequent serious adverse reactions reported were pneumonia (4.7%), upper respiratory tract infection (4.7%), basal cell carcinoma (4.7%), influenza (3.5%), general physical health deterioration (3.5%), and hypercalcemia (3.5%)1
- Permanent discontinuation of DARZALEX® due to an adverse reaction occurred in 8% of patients1
- Fatal adverse reactions within 30 days of the last dose of any study treatment occurred in 3.5% of patients who died of general physical health deterioration, multi-organ failure secondary to pulmonary aspergillosis, and disease progression1
DARZALEX® + Kd infusion-related reactions1
- Infusion-related reactions (IRRs) occurred in 41% of patients; 12% were Grade 3 or 4
- IRRs of any grade or severity may require management by interruption, modification, and/or discontinuation of the infusion
- Most IRRs occurred during the first infusion
37%
of patients had IRRs with the Week 1 infusion
2%
of patients had IRRs with the Week 2 infusion
6%
of patients had IRRs with subsequent infusions
IRRs=infusion-related reactions.
- For 37% of patients, IRRs (any grade) occurred with the Week 1 (16 mg/kg) infusion; for 2% of patients, with the Week 2 infusion; and cumulatively, for 6% of patients with subsequent infusions
- Median time to onset of an IRR was 1.5 hours (range: 0–73 hours)
- Incidence of infusion modification due to reactions was 36%
- DARZALEX® can cause severe IRRs. Severe IRRs included bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension, and blurred vision
- Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with DARZALEX® infusion. If ocular symptoms occur, interrupt DARZALEX® infusion and seek immediate ophthalmologic evaluation prior to restarting DARZALEX®
For IRRs of any grade/severity, immediately interrupt the DARZALEX® infusion and manage symptoms. Management of IRRs may further require reduction in the rate of infusion or treatment discontinuation of DARZALEX® as outlined below.1
IRR=infusion-related reaction.
DARZALEX® binds to CD38 found on red blood cells (RBCs) and results in a positive indirect antiglobulin test (indirect Coombs test) that may persist for up to 6 months after the last DARZALEX® infusion.
Reminders
- Type and screen patients before starting DARZALEX®
- Inform blood banks when a patient is on DARZALEX®
- Identify any DARZALEX®-treated blood samples
- Ask patients to tell other healthcare professionals that they've taken DARZALEX®
Brochure on daratumumab and serological testing
Identification card to give to patients taking daratumumab
An open-label, multi-cohort study
Patients had received prior treatment with a proteasome inhibitor (PI) and an immunomodulatory agent1
- This trial included 103 patients who received DARZALEX® 16 mg/kg in combination with Pd and were treated with pre- and post-infusion medications. Patients were treated until disease progression or unacceptable toxicity1
- Patients had a median of 4 prior lines of therapy1
- Of the patients who were tested for cytogenetics, 74.7% were standard risk and 25.3% were high risk7
Patients were refractory to prior therapies1*
- 89% to lenalidomide
- 71% to bortezomib
- 64% to bortezomib and lenalidomide
DPd=DARZALEX® (D) + pomalidomide (P) + dexamethasone (d); Pd=pomalidomide (P) + dexamethasone (d).
*Please note that this is not a full list of prior therapies.
Patient characteristics7
ECOG=Eastern Cooperative Oncology Group.
National Comprehensive Cancer Network® (NCCN®) Category 1, preferred
Daratumumab* (D) in combination with pomalidomide (P) and dexamethasone (d) is recommended by the NCCN Guidelines as a Category 1 preferred† therapeutic option for patients with relapsed or refractory multiple myeloma, after 2 prior therapies‡
*Daratumumab includes both daratumumab and hyaluronidase-fihj (DARZALEX FASPRO®) for subcutaneous injection and daratumumab (DARZALEX®) for intravenous infusion. Daratumumab and hyaluronidase-fihj for subcutaneous injection has different dosing and administration instructions compared to daratumumab for intravenous infusion.
†See nccn.org for definitions of NCCN Categories of Preference and NCCN Categories of Evidence and Consensus.
‡Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V3.2023. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed December 12, 2022. To view the most recent and complete version of the guideline, go online to www.nccn.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Efficacy demonstrated with DARZALEX® + Pd
CI=confidence interval; CR=complete response; ORR=overall response rate; Pd=pomalidomide (P) + dexamethasone (d); PR=partial response; sCR=stringent complete response; VGPR=very good partial response.
*Efficacy results were based on ORR as determined by an Independent Review Committee (IRC) assessment using International Myeloma Working Group (IMWG) criteria.1
Median time to response
- With DARZALEX® + Pd (n=61), median time to response was 1 month (range: 0.9 to 2.8 months)1
DARZALEX® + Pd safety profile
Adverse reactions with incidence ≥10%1
Pd=pomalidomide (P) + dexamethasone (d).
aInfusion-related reaction includes terms determined by investigators to be related to infusion.
bEdema, edema peripheral, peripheral swelling.
cAcute tonsillitis, bronchitis, laryngitis, nasopharyngitis, pharyngitis, respiratory syncytial virus infection, rhinitis, sinusitis, tonsillitis, upper respiratory tract infection.
dLung infection, pneumonia, pneumonia aspiration.
eCough, productive cough, allergic cough.
fDyspnea, dyspnea exertional.
- Infusion reaction includes terms determined by investigators to be related to infusion. Severe infusion reactions included bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension, and blurred vision1
- The most frequent adverse reactions (≥20%) with DARZALEX® + Pd (DPd) were infusion reactions, diarrhea, constipation, nausea, vomiting, fatigue, pyrexia, upper respiratory tract infection, muscle spasms, back pain, arthralgia, dizziness, insomnia, cough, and dyspnea1
- The overall incidence of serious adverse reactions was 49%. Serious adverse reactions reported in ≥5% of patients included pneumonia (7%)1
Treatment-emergent hematology laboratory abnormalities1
Pd=pomalidomide (P) + dexamethasone (d).
- The discontinuation rate due to adverse reactions with DARZALEX® + Pd was 13%1
37%
of patients had IRRs with the Week 1 infusion
2%
of patients had IRRs with the Week 2 infusion
6%
of patients had IRRs with subsequent infusions
IRRs=infusion-related reactions.
- For 37% of patients, IRRs (any grade) occurred with the Week 1 (16 mg/kg) infusion; for 2% of patients, with the Week 2 infusion; and cumulatively, for 6% of patients with subsequent infusions
- Median time to onset of an IRR was 1.5 hours (range: 0–73 hours)
- Incidence of infusion modification due to reactions was 36%
- DARZALEX® can cause severe IRRs. Severe IRRs included bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension, and blurred vision
- Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with DARZALEX® infusion. If ocular symptoms occur, interrupt DARZALEX® infusion and seek immediate ophthalmologic evaluation prior to restarting DARZALEX®
For IRRs of any grade/severity, immediately interrupt the DARZALEX® infusion and manage symptoms. Management of IRRs may further require reduction in the rate of infusion or treatment discontinuation of DARZALEX® as outlined below.1
IRR=infusion-related reaction.
DARZALEX® binds to CD38 found on red blood cells (RBCs) and results in a positive indirect antiglobulin test (indirect Coombs test) that may persist for up to 6 months after the last DARZALEX® infusion.
Reminders
- Type and screen patients before starting DARZALEX®
- Inform blood banks when a patient is on DARZALEX®
- Identify any DARZALEX®-treated blood samples
- Ask patients to tell other healthcare professionals that they've taken DARZALEX®
Brochure on daratumumab and serological testing
Identification card to give to patients taking daratumumab
A phase 2, single-arm, open-label, multicenter trial in heavily pretreated patients8
All patients received prior treatment with a proteasome inhibitor (PI) and an immunomodulatory agent.8
Patients were refractory to prior therapies1*
- 97% to the last line of treatment
- 95% to both a PI and an immunomodulatory agent
- 90% to bortezomib
- 88% to lenalidomide
- 77% to alkylating agents
*Please note that this is not a full list of prior therapies.
Patient characteristics
ECOG=Eastern Cooperative Oncology Group.
This trial included 106 patients with relapsed or refractory multiple myeloma who were administered pre- and post-infusion medications and treated with DARZALEX® 16 mg/kg until unacceptable toxicity or disease progression.1
National Comprehensive Cancer Network® (NCCN®) Category 2A, useful in certain circumstances
Daratumumab* (D) monotherapy is recommended by the NCCN Guidelines as a Category 2A, useful in certain circumstances† therapeutic option for patients with relapsed or refractory multiple myeloma, after 3 prior therapies‡
*Daratumumab includes both daratumumab and hyaluronidase-fihj (DARZALEX FASPRO®) for subcutaneous injection and daratumumab (DARZALEX®) for intravenous infusion. Daratumumab and hyaluronidase-fihj for subcutaneous injection has different dosing and administration instructions compared to daratumumab for intravenous infusion.
†See nccn.org for definitions of NCCN Categories of Preference and NCCN Categories of Evidence and Consensus.
‡Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V3.2023. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed December 12, 2022. To view the most recent and complete version of the guideline, go online to www.nccn.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Overall response rate (ORR) with DARZALEX® monotherapy1*
CI=confidence interval; ORR=overall response rate; PR=partial response; sCR=stringent complete response; VGPR=very good partial response.
- DARZALEX® achieved sCR + VGPR in 12% of patients1
*Efficacy results were based on ORR as determined by an Independent Review Committee (IRC) assessment using International Myeloma Working Group (IMWG) criteria.1
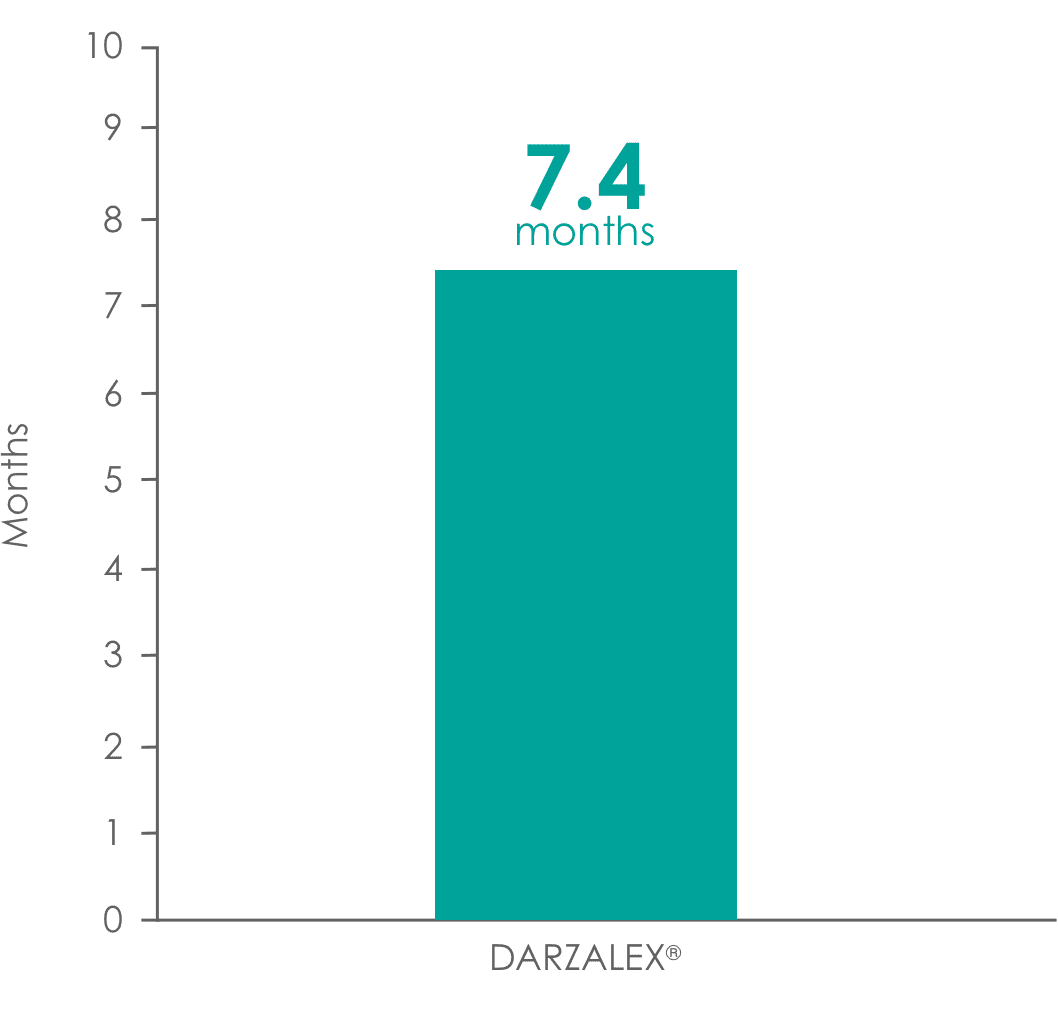
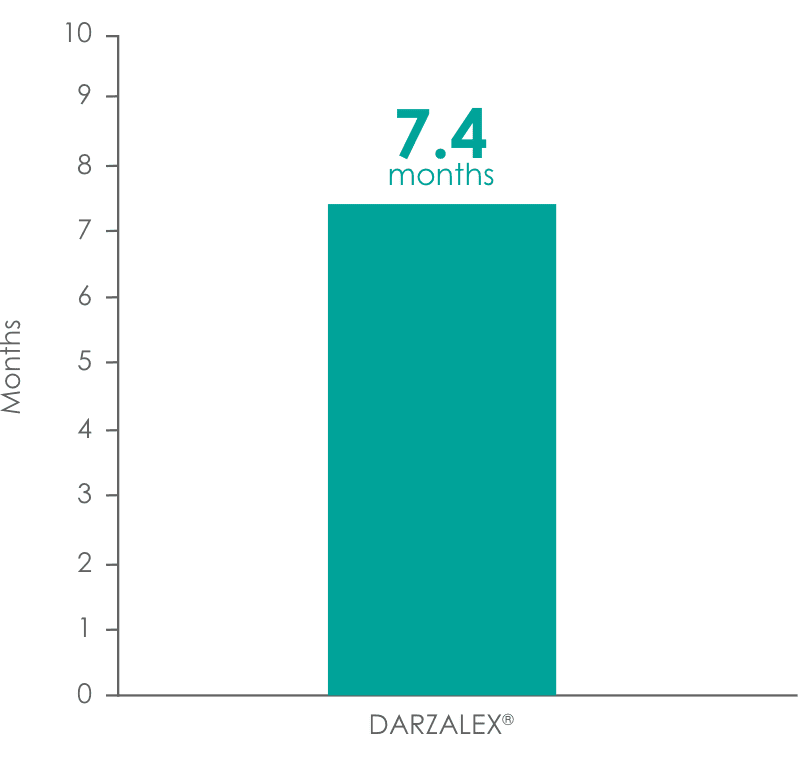
DARZALEX® provided a 7.4-month median duration of response (range: 1.2 to 13.1+ months)1
Median duration of response
DARZALEX® monotherapy safety profile
Adverse reactions with incidence ≥10%1
aInfusion-related reaction includes terms determined by investigators to be related to infusion.
bPneumonia also includes the terms streptococcal pneumonia and lobar pneumonia.
- Infusion reaction includes terms determined by investigators to be related to infusion. Severe infusion reactions included bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension, and blurred vision1
- Safety data were pooled from 3 open-label clinical studies of relapsed or refractory patients treated with DARZALEX® 16 mg/kg (n=156)1
Treatment emergent Grade 3/4 laboratory abnormalities (≥10%)1
37%
of patients had IRRs with the Week 1 infusion
2%
of patients had IRRs with the Week 2 infusion
6%
of patients had IRRs with subsequent infusions
IRRs=infusion-related reactions.
- For 37% of patients, IRRs (any grade) occurred with the Week 1 (16 mg/kg) infusion; for 2% of patients, with the Week 2 infusion; and cumulatively, for 6% of patients with subsequent infusions
- Median time to onset of an IRR was 1.5 hours (range: 0–73 hours)
- Incidence of infusion modification due to reactions was 36%
- DARZALEX® can cause severe IRRs. Severe IRRs included bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension, and blurred vision
- Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with DARZALEX® infusion. If ocular symptoms occur, interrupt DARZALEX® infusion and seek immediate ophthalmologic evaluation prior to restarting DARZALEX®
For IRRs of any grade/severity, immediately interrupt the DARZALEX® infusion and manage symptoms. Management of IRRs may further require reduction in the rate of infusion or treatment discontinuation of DARZALEX® as outlined below.1
IRR=infusion-related reaction.
DARZALEX® binds to CD38 found on red blood cells (RBCs) and results in a positive indirect antiglobulin test (indirect Coombs test) that may persist for up to 6 months after the last DARZALEX® infusion.
Reminders
- Type and screen patients before starting DARZALEX®
- Inform blood banks when a patient is on DARZALEX®
- Identify any DARZALEX®-treated blood samples
- Ask patients to tell other healthcare professionals that they've taken DARZALEX®
Brochure on daratumumab and serological testing
Identification card to give to patients taking daratumumab
DARZALEX FASPRO® combination therapy trial in newly diagnosed multiple myeloma
- DVMP: eligible patients were required to have newly diagnosed multiple myeloma who are ineligible for transplant. The median age was 75 years old (range: 66–86 years): 46% were male; 69% were White, 8% Asian, and 2% Black or African American; 33% had International Staging System (ISS) Stage I, 45% had ISS Stage II, and 22% had ISS Stage III disease9
DARZALEX FASPRO® combination therapy trial in relapsed or refractory multiple myeloma
- DRd: the median age of patients was 69 years old (range: 33-82 years); 69% were male; 69% were White, and 3% Black or African American. Forty-two percent had ISS Stage I, 30% had ISS Stage II, and 28% had ISS Stage III disease. Patients had received a median of 1 prior line of therapy: 52% had a prior autologous
stem-cell transplant (ASCT); 95% had received a prior proteasome inhibitor (PI); 59% had received a prior immunomodulatory agent, including 22% who received prior lenalidomide; and 54% of patients received both a prior PI and an immunomodulatory agent9
- Gastrointestinal disorders: abdominal pain, constipation, pancreatitis
- Infection and infestations: pneumonia, influenza, urinary tract infection, herpes zoster, sepsis
- Metabolism and nutrition disorders: hyperglycemia, decreased appetite, hypocalcemia
- Musculoskeletal and connective tissue disorders: muscle spasms, arthralgia
- Nervous system disorders: paresthesia, dizziness, syncope
- General disorders and administration site conditions: injection site reaction, infusion reactions, chills
- Skin and subcutaneous tissue disorders: rash, pruritus
- Cardiac disorders: cardiac failure
- Vascular disorders: hypotension
- The median age was 67 years (range: 35–90 years); 53% were male and 89% were White, <1% were Black or African American, and <1% were Asian
- 45% had International Staging System (ISS) Stage I, 33% had ISS Stage II, and 22% had ISS Stage III disease
- Patients had received a median of 2 prior lines of therapy (range: 1–5), with 11% of patients having received 1 prior line of therapy and 75% of patients having received 2 to 3 prior lines of therapy
- All patients received a prior treatment with a proteasome inhibitor (PI) and lenalidomide, and 56% of patients received prior autologous stem cell transplant (ASCT)
- The majority of patients were refractory to lenalidomide (80%), a PI (48%), or both an immunomodulatory agent and a PI (42%)
- The most common adverse reactions (≥20%) were fatigue, pneumonia, upper respiratory tract infection, and diarrhea9
- Serious adverse reactions occurred in 50% of patients who received DARZALEX FASPRO® + Pd. The most frequent serious adverse reactions in >5% of patients who received DARZALEX FASPRO® + Pd were pneumonia (15%) and lower respiratory tract infection (12%). Fatal adverse reactions occurred in 7% of patients who received DARZALEX FASPRO® + Pd9
- Permanent treatment discontinuation due to an adverse reaction occurred in 2% of patients who received DARZALEX FASPRO® + Pd9