For newly diagnosed, transplant-ineligible multiple myeloma, ALCYONE trial studied the addition of DARZALEX® to VMP regimen1
A large, randomized, open-label, multicenter, active-controlled phase 3 trial of DARZALEX® + VMP vs VMP alone1,2


- Patients in the DARZALEX® + VMP arm received DARZALEX® 16 mg/kg until disease progression or unacceptable toxicity1
- Participants received bortezomib 1.3 mg/m2 as subcutaneous injection, twice weekly at Weeks 1, 2, 4, and 5 (Cycle 1) followed by once weekly at Weeks 1, 2, 4, and 5 (Cycles 2-9); melphalan 9 mg/m2 and prednisone 60 mg/m2 were orally administered on Days 1 to 4 of the nine 6-week cycles (Cycles 1-9). Per protocol, VMP treatment was discontinued in both arms after 9 cycles*1
- Primary endpoint was efficacy evaluated by progression-free survival (PFS) based on International Myeloma Working Group (IMWG) criteria2
- Key secondary endpoints included overall response rate (ORR), rates of very good partial response (VGPR) or better, complete response (CR) or better, negative status for minimal residual disease (MRD), and overall survival (OS)2
- Additional endpoints included time to response, duration of response, safety, and side effect profile2
ASCT=autologous stem cell transplant; DVMP=DARZALEX® (D) + bortezomib (V) + melphalan (M) + prednisone (P); ECOG PS=Eastern Cooperative Oncology Group Performance Status; VMP=bortezomib (V) + melphalan (M) + prednisone (P).
*Prednisone was withheld on Day 1 of each cycle when dexamethasone was used as premedication to reduce the risk of infusion reactions.2
Patient characteristics: baseline demographics were similar between arms1,2


ECOG PS=Eastern Cooperative Oncology Group Performance Status; VMP=bortezomib (V) + melphalan (M) + prednisone (P).
National Comprehensive Cancer Network® (NCCN®) Category 1, other recommended
Daratumumab* (D) in combination with bortezomib (V), melphalan (M), and prednisone (P) is recommended by the NCCN Guidelines as a Category 1, other recommended† therapeutic option for patients with newly diagnosed, transplant-ineligible multiple myeloma‡
*Daratumumab includes both daratumumab (DARZALEX®) for intravenous infusion and daratumumab and hyaluronidase-fihj (DARZALEX FASPRO®) for subcutaneous injection.
†See NCCN.org for definitions of NCCN Categories of Preference and NCCN Categories of Evidence and Consensus.
‡Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V.2.2024. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed November 2, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
DARZALEX® + VMP demonstrated proven efficacy vs VMP alone1
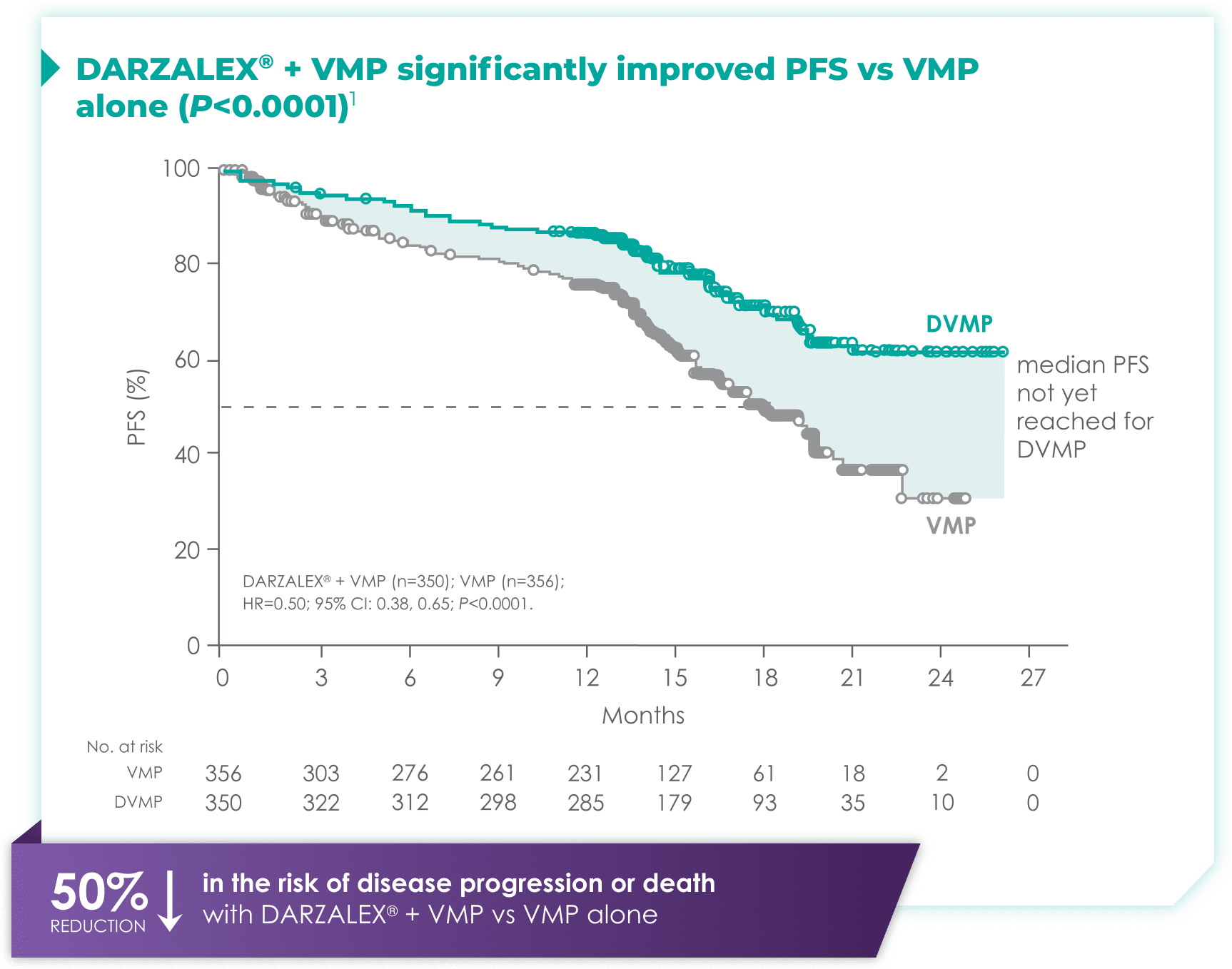

CI=confidence interval; DVMP=DARZALEX® (D) + bortezomib (V) + melphalan (M) + prednisone (P); HR=hazard ratio; PFS=progression-free survival; VMP=bortezomib (V) + melphalan (M) + prednisone (P).
Median PFS in the DARZALEX® + VMP regimen had not been reached vs 18.1 months with VMP alone1
DARZALEX® + VMP significantly improved OS vs VMP alone (P=0.0003)1


CI=confidence interval; DVMP=DARZALEX® (D) + bortezomib (V) + melphalan (M) + prednisone (P); HR=hazard ratio; OS=overall survival; VMP=bortezomib (V) + melphalan (M) + prednisone (P).
Median OS was not reached for either arm1
Significant improvement in ORR with DARZALEX® + VMP1
Almost all patients (91%) treated with DARZALEX® + VMP demonstrated a clinical response.1


CR=complete response; ORR=overall response rate; PR=partial response; sCR=stringent complete response; VGPR=very good partial response; VMP=bortezomib (V) + melphalan (M) + prednisone (P).
Speed of response
- In responders, median time to response was 0.79 months (range: 0.4-15.5 months) in the DARZALEX® + VMP group and 0.82 months (range: 0.7-12.6 months) in the VMP group1
Depth of response
- 42.6% of patients achieved CR or better with DARZALEX® + VMP vs 24.4% with VMP alone1
Duration of response
- Median duration of response had not yet been reached with DARZALEX® + VMP vs 21.3 months (range: 0.5+, 23.7+) with VMP alone1
Significantly higher MRD negativity rate with DARZALEX® + VMP1
3.6x higher MRD negativity rate with DARZALEX® + VMP vs VMP alone (P<0.0001).

DARZALEX® + VMP
(n=78)
(95% CI: 18.0, 27.0)
(n=78)
(95% CI: 18.0, 27.0)
VS

with VMP alone
(n=22)
(95% CI: 3.9, 9.2)
(n=22)
(95% CI: 3.9, 9.2)
- Minimal residual disease (MRD) based on threshold of 10-5 using a next-generation sequencing assay
- In patients with CR or better, the MRD negativity rate was 49.7% (n=74) (95% CI: 41.4, 58.0) in the DARZALEX® + VMP arm vs 25.3% (n=22) (95% CI: 16.6, 35.7) with VMP arm
DARZALEX® + VMP safety profile
Adverse reactions with incidence ≥10%1


VMP=bortezomib (V) + melphalan (M) + prednisone (P).
aUpper respiratory tract infection, bronchitis, bronchitis bacterial, epiglottitis, laryngitis, laryngitis bacterial, metapneumovirus infection, nasopharyngitis, oropharyngeal candidiasis, pharyngitis, pharyngitis streptococcal, respiratory syncytial virus infection, respiratory tract infection, respiratory tract infection viral, rhinitis, sinusitis, tonsillitis, tracheitis, tracheobronchitis, viral pharyngitis, viral rhinitis, viral upper respiratory tract infection.
bInfusion-related reaction includes terms determined by investigators to be related to infusion.
cEdema peripheral, generalized edema, peripheral swelling.
dPneumonia, lung infection, pneumonia aspiration, pneumonia bacterial, pneumonia pneumococcal, pneumonia streptococcal, pneumonia viral, and pulmonary sepsis.
eCough, productive cough.
fDyspnea, dyspnea exertional.
gHypertension, blood pressure increased.
Note: Adverse reactions that occurred in ≥10% of patients and with at least 5% greater frequency in the DARZALEX® + VMP arm are listed.1
- The most frequent adverse reactions (≥20% with at least 5% greater frequency in the DVMP arm) were infusion reactions, upper respiratory tract infection, and peripheral edema1
- Serious adverse reactions with at least a 2% greater incidence in the DARZALEX® + VMP arm compared with the VMP arm were pneumonia (DVMP 11% vs VMP 4%), upper respiratory tract infection (DVMP 5% vs VMP 1%), and pulmonary edema (DVMP 2% vs VMP 0%)1
Treatment-emergent hematologic laboratory abnormalities1


VMP=bortezomib (V) + melphalan (M) + prednisone (P).
- Grade 3 or 4 infections were 23% with DARZALEX® + VMP vs 15% with VMP alone2
Discontinuation rates due to adverse reactions with DARZALEX® combination therapy in newly diagnosed, transplant-ineligible patients with multiple myeloma2

DARZALEX® + VMP
VS

with VMP alone
Frequency of IRRs (any grade) across clinical trials (N=2,066)1


IRR=infusion-related reaction.
Most IRRs occurred during the first infusion across clinical trials (N=2,066)1
- For 37% of patients, IRRs (any grade) occurred with the Week 1 (16 mg/kg) infusion; for 2% of patients, with the Week 2 infusion; and cumulatively, for 6% of patients with subsequent infusions
- Median time to onset of an IRR was 1.5 hours (range: 0–73 hours)
- Incidence of infusion modification due to reactions was 36%
- DARZALEX® can cause severe IRRs. Severe IRRs included bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension, and blurred vision
- Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with DARZALEX® infusion. If ocular symptoms occur, interrupt DARZALEX® infusion and seek immediate ophthalmologic evaluation prior to restarting DARZALEX®
Management of IRRs
For IRRs of any grade/severity, immediately interrupt the DARZALEX® infusion and manage symptoms. Management of IRRs may further require reduction in the rate of infusion or treatment discontinuation of DARZALEX® as outlined below.1


IRR=infusion-related reaction.
Interference with serological testing1
DARZALEX® binds to CD38 found on red blood cells (RBCs) and results in a positive indirect antiglobulin test (indirect Coombs test) that may persist for up to 6 months after the last DARZALEX® infusion.
Reminders
- Type and screen patients before starting DARZALEX®
- Inform blood banks when a patient is on DARZALEX®
- Identify any blood samples of patients treated with DARZALEX®
- Ask patients to tell other healthcare professionals that they've taken DARZALEX®
Neutropenia and thrombocytopenia1
DARZALEX® may increase neutropenia and thrombocytopenia induced by background therapy. Monitor complete blood cell counts periodically during treatment according to manufacturer’s prescribing information for background therapies. Monitor patients with neutropenia for signs of infection. Consider withholding DARZALEX® until recovery of neutrophils or for recovery of platelets.
Interference with determination of complete response1
Daratumumab is a human immunoglobulin G (IgG) kappa monoclonal antibody that can be detected on both the serum protein electrophoresis (SPE) and immunofixation (IFE) assays used for the clinical monitoring of endogenous M-protein. This interference can impact the determination of complete response and of disease progression in some patients with IgG kappa myeloma protein.
Embryo-fetal toxicity1
Based on the mechanism of action, DARZALEX® can cause fetal harm when administered to a pregnant woman. DARZALEX® may cause depletion of fetal immune cells and decreased bone density. Advise pregnant women of the potential risk to a fetus. Advise females with reproductive potential to use effective contraception during treatment with DARZALEX® and for 3 months after the last dose.
The combination of DARZALEX® with lenalidomide, pomalidomide, or thalidomide is contraindicated in pregnant women because lenalidomide, pomalidomide, and thalidomide may cause birth defects and death of the unborn child. Refer to the lenalidomide, pomalidomide, or thalidomide prescribing information on use during pregnancy.
Brochure on daratumumab and serological testing
Identification card to give to patients taking daratumumab
For newly diagnosed, transplant-eligible multiple myeloma, CASSIOPEIA trial demonstrated significance of adding DARZALEX® to VTd regimen1
Phase 3 study comparing DARZALEX® + VTd vs VTd alone for induction and consolidation treatment1,2


- In an ongoing part 2 of the study, patients underwent a second randomization in both treatment groups to either observation or DARZALEX® Q8W monotherapy for maintenance. The efficacy and safety of this maintenance regimen is currently being evaluated2
- DARZALEX® dosing and administration: IV, 16 mg/kg actual body weight QW Cycles 1–2 and Q2W Cycles 3–4 (induction phase); Q2W Cycles 5–6 (consolidation phase)1
- VTd dosing and administration: Bortezomib (V) SC or IV, 1.3 mg/m2 BSA twice weekly for 2 weeks on Days 1, 4, 8, and 11 of repeated 28-day (4-week) induction cycles (Cycles 1–4) and 2 consolidation cycles (Cycles 5–6); thalidomide (T) PO, 100 mg daily during the 6 bortezomib cycles; dexamethasone (d) PO or IV, 40 mg on Days 1, 2, 8, 9, 15, 16, 22, and 23 of Cycles 1 and 2, and at 40 mg on Days 1–2 and 20 mg on subsequent dosing days (Days 8, 9, 15, 16) of Cycles 3–4. Dexamethasone 20 mg was administered on Days 1, 2, 8, 9, 15, and 16 in Cycles 5–6. On the days of DARZALEX® infusion, the dexamethasone dose was administered intravenously as a pre-infusion medication1
- Primary endpoint was stringent complete response (sCR) rate at Day 100 post-transplant2
- Key secondary endpoints included: rate of CR or better, PFS from first randomization and OS from first randomization2
- Patient characteristics: the median age was 58 years; 59% male; 48% had an ECOG PS of 0, 42% had an ECOG PS of 1, and 10% had an ECOG PS of 2; 40% had ISS Stage I, 45% had ISS Stage II, and 15% had ISS Stage III disease1
- Approval of frontline treatment of transplant-eligible patients with multiple myeloma is based on the response evaluation of Part 1 only of this trial at Day 100 post-transplant
BSA=body surface area; CR=complete response; DVTd=DARZALEX® (D) + bortezomib (V) + thalidomide (T) + dexamethasone (d); ECOG PS=Eastern Cooperative Oncology Group Performance Status; ISS=International Staging System; IV=intravenous; OS=overall survival; PD=progressive disease; PFS=progression-free survival; PO=by mouth; PR=partial response; QW=weekly; Q2W=every 2 weeks; Q8W=every 8 weeks; SC=subcutaneous; VTd=bortezomib (V) + thalidomide (T) + dexamethasone (d).
National Comprehensive Cancer Network® (NCCN®) Category 2A, useful in certain circumstances
Daratumumab* (D) in combination with bortezomib (V), thalidomide (T) and dexamethasone (d) is recommended by the NCCN Guidelines as a Category 2A, useful in certain circumstances† therapeutic option for newly diagnosed, transplant-eligible multiple myeloma‡
*Daratumumab includes both daratumumab (DARZALEX®) for intravenous infusion and daratumumab and hyaluronidase-fihj (DARZALEX FASPRO®) for subcutaneous injection.
†See NCCN.org for definitions of NCCN Categories of Preference and NCCN Categories of Evidence and Consensus.
‡Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V.2.2024. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed November 2, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Adding DARZALEX® to VTd resulted in significantly more patients with sCR vs VTd alone after consolidation1,2


DARZALEX® + VTd post-consolidation responses1,2


CR=complete response; ITT=intent-to-treat; ORR=overall response rate; PR=partial response; sCR=stringent complete response; VGPR=very good partial response; VTd=bortezomib (V) + thalidomide (T) + dexamethasone (d).
53% reduction in the risk of disease progression or death with DARZALEX® + VTd vs VTd alone*1


*Based on interim analysis and the boundary for PFS was crossed.
~90% of patients in each treatment arm underwent ASCT2
- 489 (90%) with DARZALEX® + VTd and 484 (89%) with VTd alone2
- 85% of patients in the DARZALEX® + VTd group and 81% of those in the VTd group completed all 4 induction and both consolidation cycles at 18.8 months (median follow-up)1,2
ASCT=autologous stem cell transplant; CI=confidence interval; HR=hazard ratio; PFS=progression-free survival; VTd=bortezomib (V) + thalidomide (T) + dexamethasone (d).
DARZALEX® + VTd safety profile for induction and consolidation treatment1
Most frequent adverse reactions and laboratory abnormalities reported in ≥20% of patients and with at least a 5% greater frequency in the DARZALEX® + VTd arm.*1


VTd=bortezomib (V) + thalidomide (T) + dexamethasone (d).
aInfusion-related reaction includes terms determined by investigators to be related to infusion.
bLaryngitis, laryngitis viral, metapneumovirus infection, nasopharyngitis, oropharyngeal candidiasis, pharyngitis, respiratory syncytial virus infection, respiratory tract infection, respiratory tract infection viral, rhinitis, rhinovirus infection, sinusitis, tonsillitis, tracheitis, upper respiratory tract infection, viral pharyngitis, viral rhinitis, viral upper respiratory tract infection.
cBronchiolitis, bronchitis, bronchitis chronic, respiratory syncytial virus bronchitis, tracheobronchitis.


VTd=bortezomib (V) + thalidomide (T) + dexamethasone (d).
*Adverse reactions that occurred with a frequency of ≥10% and <20%, and with at least a 5% greater frequency in the DARZALEX® + VTd arm were cough, vomiting, and hypertension.1
Serious adverse reactions with a 2% greater incidence in the DVTd arm compared with the VTd arm were bronchitis (DVTd 2% vs VTd <1%) and pneumonia (DVTd 6% vs VTd 4%).1
- Discontinuation rates due to any adverse event: 7% with DVTd vs 8% with VTd2
- Infusion reactions with DVTd occurred in 35% of patients; 3% were Grade 3 and <1% were Grade 41
- Most infusion reactions occurred during the first infusion2
Frequency of infusion reactions (any grade) in the clinical trial (n=536)2


IR=infusion reaction.
†Overall, 12% of patients had infusion reactions with subsequent infusions.2
- Administer pre-infusion and post-infusion medications to reduce the risk of infusion reactions [see section 2.3 of the DARZALEX® full Prescribing Information]1
DVTd=DARZALEX® (D) + bortezomib (V) + thalidomide (T) + dexamethasone (d); VTd=bortezomib (V) + thalidomide (T) + dexamethasone (d).
Frequency of IRRs (any grade) across clinical trials (N=2,066)1


IRR=infusion-related reaction.
Most IRRs occurred during the first infusion across clinical trials (N=2,066)1
- For 37% of patients, IRRs (any grade) occurred with the Week 1 (16 mg/kg) infusion; for 2% of patients, with the Week 2 infusion; and cumulatively, for 6% of patients with subsequent infusions
- Median time to onset of an IRR was 1.5 hours (range: 0–73 hours)
- Incidence of infusion modification due to reactions was 36%
- DARZALEX® can cause severe IRRs. Severe IRRs included bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension, and blurred vision
- Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with DARZALEX® infusion. If ocular symptoms occur, interrupt DARZALEX® infusion and seek immediate ophthalmologic evaluation prior to restarting DARZALEX®
Management of IRRs
For IRRs of any grade/severity, immediately interrupt the DARZALEX® infusion and manage symptoms. Management of IRRs may further require reduction in the rate of infusion or treatment discontinuation of DARZALEX® as outlined below.1


IRR=infusion-related reaction.
Interference with serological testing1
DARZALEX® binds to CD38 found on red blood cells (RBCs) and results in a positive indirect antiglobulin test (indirect Coombs test) that may persist for up to 6 months after the last DARZALEX® infusion.
Reminders
- Type and screen patients before starting DARZALEX®
- Inform blood banks when a patient is on DARZALEX®
- Identify any blood samples of patients treated with DARZALEX®
- Ask patients to tell other healthcare professionals that they've taken DARZALEX®
Neutropenia and thrombocytopenia1
DARZALEX® may increase neutropenia and thrombocytopenia induced by background therapy. Monitor complete blood cell counts periodically during treatment according to manufacturer’s prescribing information for background therapies. Monitor patients with neutropenia for signs of infection. Consider withholding DARZALEX® until recovery of neutrophils or for recovery of platelets.
Interference with determination of complete response1
Daratumumab is a human immunoglobulin G (IgG) kappa monoclonal antibody that can be detected on both the serum protein electrophoresis (SPE) and immunofixation (IFE) assays used for the clinical monitoring of endogenous M-protein. This interference can impact the determination of complete response and of disease progression in some patients with IgG kappa myeloma protein.
Embryo-fetal toxicity1
Based on the mechanism of action, DARZALEX® can cause fetal harm when administered to a pregnant woman. DARZALEX® may cause depletion of fetal immune cells and decreased bone density. Advise pregnant women of the potential risk to a fetus. Advise females with reproductive potential to use effective contraception during treatment with DARZALEX® and for 3 months after the last dose.
The combination of DARZALEX® with lenalidomide, pomalidomide, or thalidomide is contraindicated in pregnant women because lenalidomide, pomalidomide, and thalidomide may cause birth defects and death of the unborn child. Refer to the lenalidomide, pomalidomide, or thalidomide prescribing information on use during pregnancy.
Brochure on daratumumab and serological testing
Identification card to give to patients taking daratumumab
NCCN Guidelines recommendations for daratumumab-containing regimens