More FDA-approved regimens for DARZALEX FASPRO® and DARZALEX® than any other monoclonal antibody in multiple myeloma1-5
Newly diagnosed
Transplant ineligible
DARZALEX® + VMP/
DARZALEX FASPRO® + VMP
Transplant eligible
DARZALEX® + VTd/
DARZALEX FASPRO® + VTd
Relapsed or refractory
After ≥1 prior therapy
DARZALEX FASPRO® + Pd
Prior line of therapy including lenalidomide and a proteasome inhibitor (PI).
After 1-3 prior lines of therapy
After ≥2 prior therapies
After ≥3 prior therapies
DARZALEX® monotherapy/
DARZALEX FASPRO® monotherapy
Prior therapies included a PI and an immunomodulatory agent, or patients were double refractory to a PI and an immunomodulatory agent.
Non-inferiority of DARZALEX FASPRO® vs DARZALEX® IV was established in COLUMBA trial1
DKd=DARZALEX FASPRO®/DARZALEX® (D) + carfilzomib (K) + dexamethasone (d); DPd=DARZALEX FASPRO®/DARZALEX® (D) + pomalidomide (P) + dexamethasone (d); DRd=DARZALEX FASPRO®/DARZALEX® (D) + lenalidomide (R) + dexamethasone (d); DVd=DARZALEX FASPRO®/DARZALEX® (D) + bortezomib (V) + dexamethasone (d); DVMP=DARZALEX FASPRO®/DARZALEX® (D) + bortezomib (V) + melphalan (M) + prednisone (P); DVRd=DARZALEX FASPRO® (D) + bortezomib (V) + lenalidomide (R) + dexamethasone (d); DVTd=DARZALEX FASPRO®/DARZALEX® (D) + bortezomib (V) + thalidomide (T) + dexamethasone (d); IV=intravenous; Kd=carfilzomib (K) + dexamethasone (d); Pd=pomalidomide (P) + dexamethasone (d); Rd=lenalidomide (R) + dexamethasone (d); Vd=bortezomib (V) + dexamethasone (d); VMP=bortezomib (V) + melphalan (M) + prednisone (P); VTd=bortezomib (V) + thalidomide (T) + dexamethasone (d).
National Comprehensive Cancer Network® (NCCN®) Guidelines recommendations for daratumumab-containing regimens
Dosing schedules for DARZALEX FASPRO® and DARZALEX®
Dosing and administration guide for DARZALEX FASPRO® and DARZALEX®
For more detailed information on dosing and administration, including management of infusion-related
reactions (IRRs) and administration-related reactions (ARRs), please click the link below.
reactions (IRRs) and administration-related reactions (ARRs), please click the link below.
Once-monthly dosing over time—a key milestone for patients to reach in newly diagnosed, transplant-ineligible multiple myeloma1,2*
Starting at Cycle 7, patients will transition to approximately once-monthly dosing for DARZALEX FASPRO® or DARZALEX®
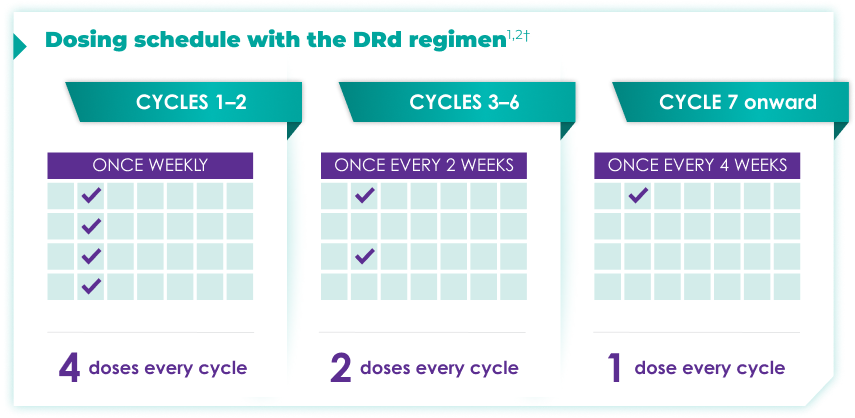
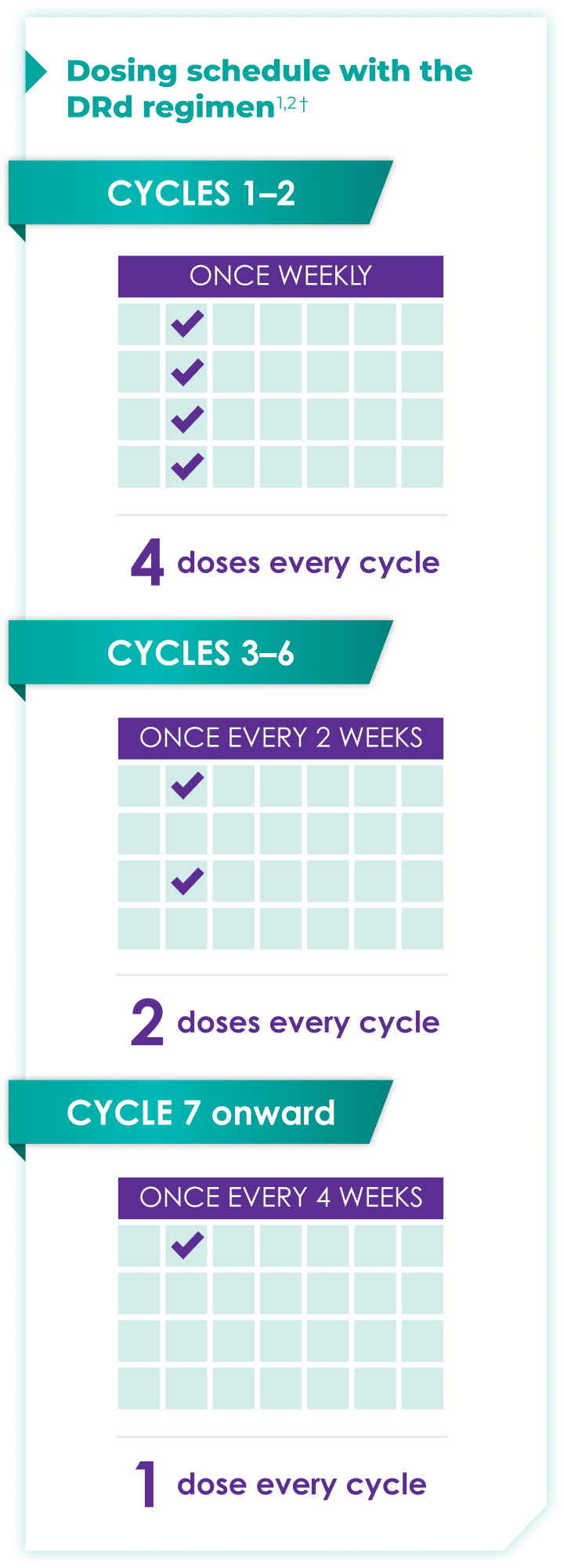
Continue DRd until disease progression or unacceptable toxicity
DRd=DARZALEX FASPRO®/DARZALEX® (D) + lenalidomide (R) + dexamethasone (d).
DARZALEX FASPRO® in combination with lenalidomide and dexamethasone (4-week cycle) has the same dosing schedule as DARZALEX® in combination with lenalidomide and dexamethasone.1,2‡
*The first dose of the every-2-week dosing schedule is given at Week 9. First dose of the every-4-week dosing schedule is given at Week 25.1,2
See the Dosage and Administration section of the full Prescribing Information for more details. When DARZALEX FASPRO® or DARZALEX® is administered as part of a combination therapy, see the Prescribing Information for dosage recommendations for the other drugs.
†Cycle=28 days.
‡The option of splitting the first dose for DARZALEX® is not applicable to DARZALEX FASPRO®.1,2
Once-monthly dosing over time—a key milestone for patients to reach in newly diagnosed, transplant-ineligible multiple myeloma1,2*
Starting at Cycle 10, patients will transition to approximately once-monthly dosing for DARZALEX FASPRO® or DARZALEX®
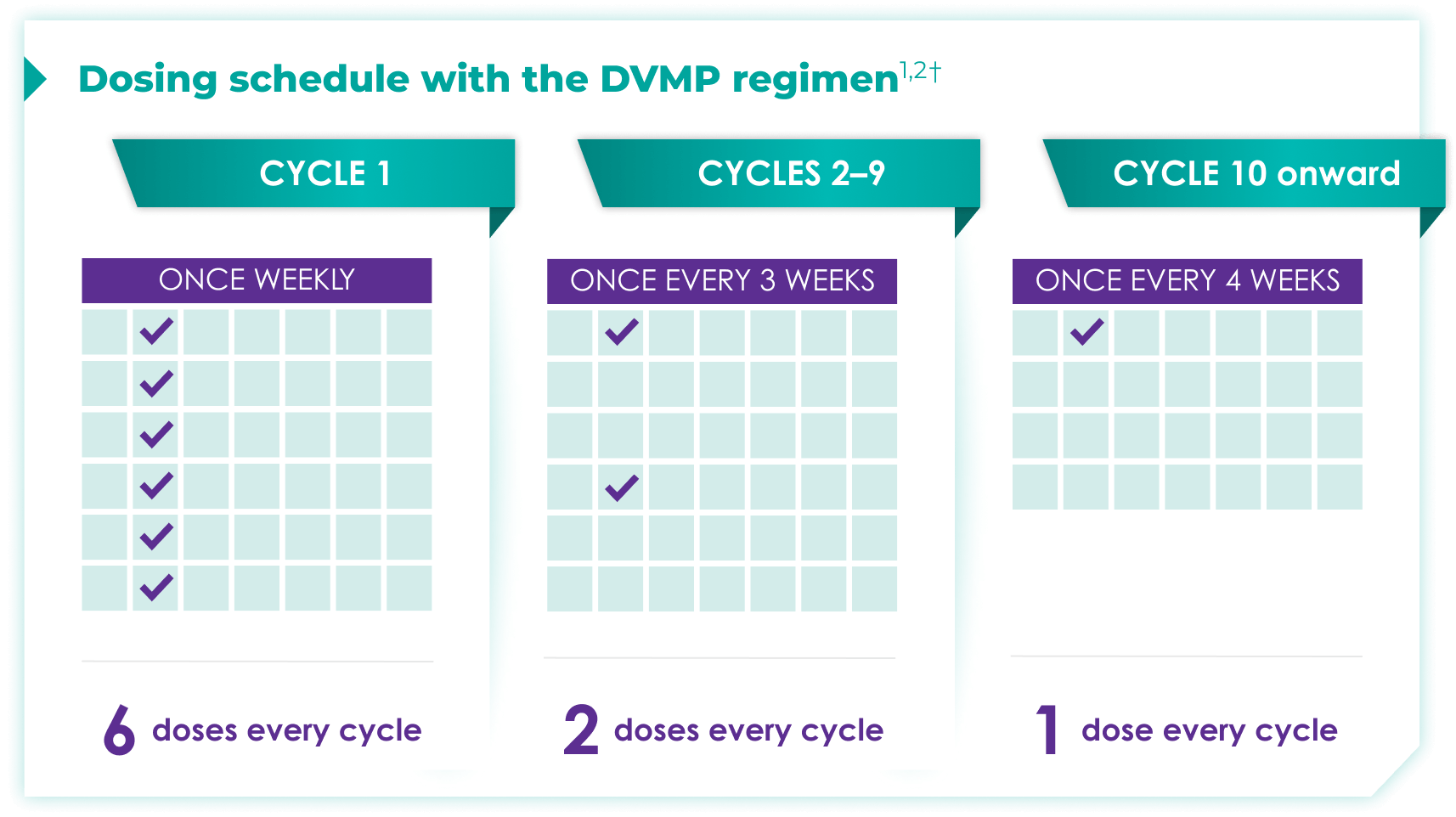
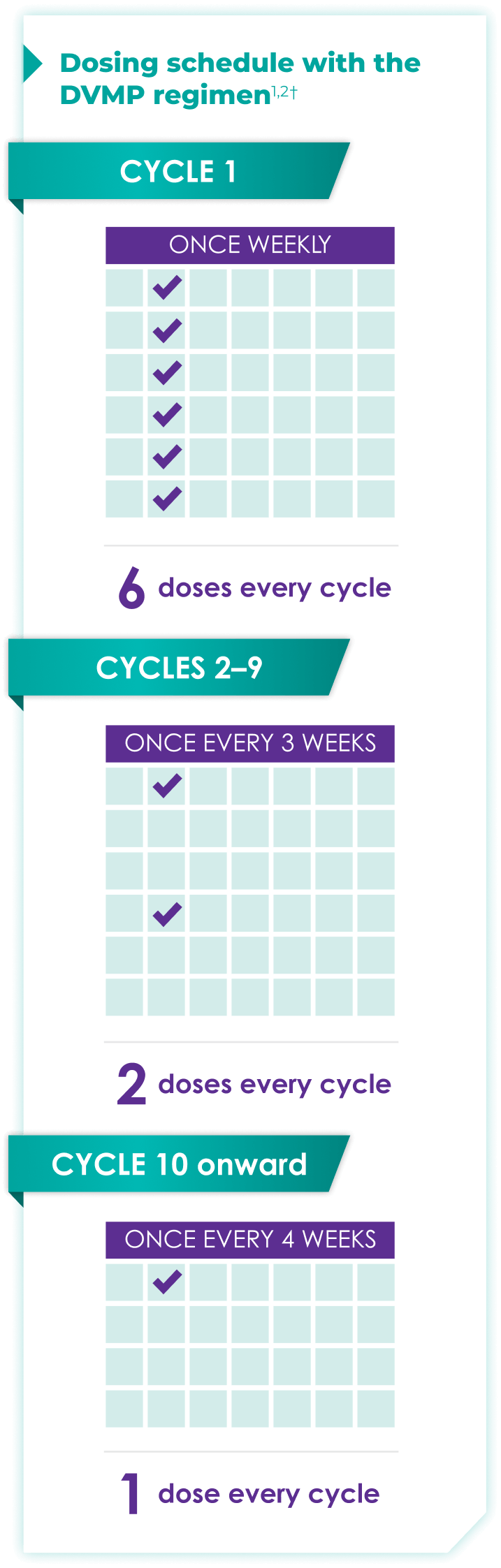
Continue DVMP until disease progression or unacceptable toxicity
DVMP=DARZALEX FASPRO®/DARZALEX® (D) + bortezomib (V) + melphalan (M) + prednisone (P).
DARZALEX FASPRO® in combination with bortezomib + melphalan + prednisone (6-week cycle) has the same dosing schedule as DARZALEX® in combination with bortezomib + melphalan + prednisone.1,2‡
*The first dose of the every-2-week dosing schedule is given at Week 9. First dose of the every-4-week dosing schedule is given at Week 25.1,2
See the Dosage and Administration section of the full Prescribing Information for more details. When DARZALEX FASPRO® or DARZALEX® is administered as part of a combination therapy, see the Prescribing Information for dosage recommendations for the other drugs.
†Cycle=28 days.
‡The option of splitting the first dose for DARZALEX® is not applicable to DARZALEX FASPRO®.1,2
Fixed-cycle dosing for patients in newly diagnosed,
transplant-eligible multiple myeloma1,2*
transplant-eligible multiple myeloma1,2*
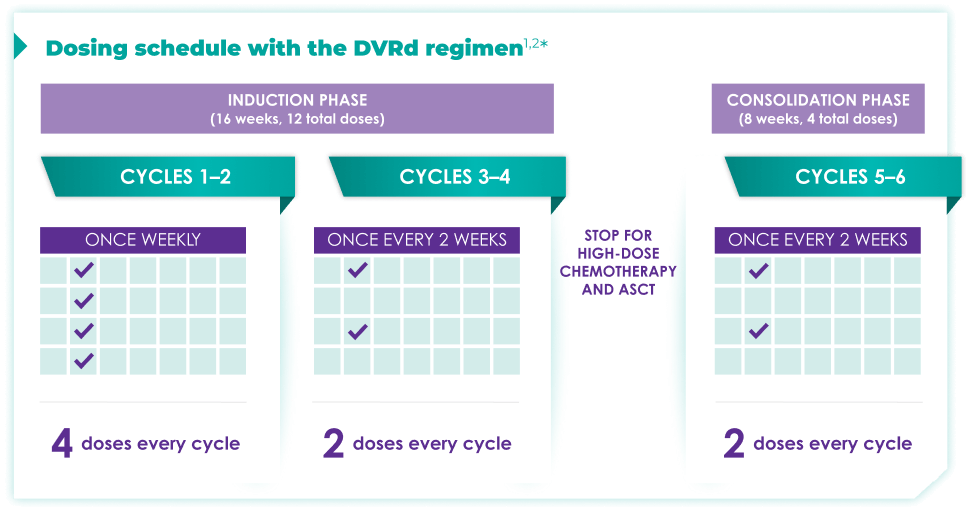
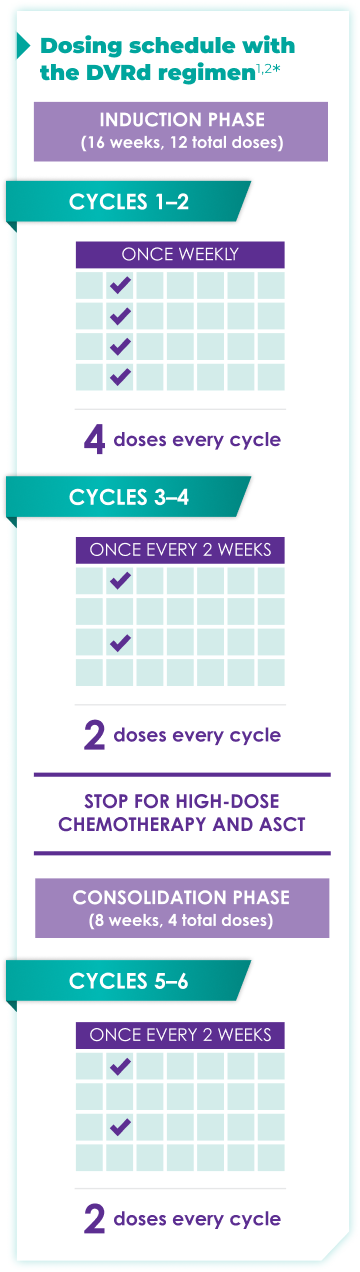
DARZALEX FASPRO® is the only anti-CD38 monoclonal antibody
that can be delivered subcutaneously in ~3 to 5 minutes.1-3
that can be delivered subcutaneously in ~3 to 5 minutes.1-3
ASCT=autologous stem cell transplant; d=dexamethasone (d); D=DARZALEX FASPRO® (D); DR=DARZALEX FASPRO® (D) + lenalidomide (R); DVRd=DARZALEX FASPRO® (D) + bortezomib (V) + lenalidomide (R) + dexamethasone (d).
DARZALEX FASPRO® in combination with bortezomib + lenalidomide and dexamethasone(4-week cycle) has the same dosing schedule as DARZALEX® in combination with bortezomib + lenalidomide and dexamethasone.1,2‡
*The first dose of the every-2-week dosing schedule is given at Week 9. First dose of the every-4-week dosing schedule is given at Week 25.1,2 See the Dosage and Administration section of the full Prescribing Information for more details. When DARZALEX FASPRO® or DARZALEX® is administered as part of a combination therapy, see the Prescribing Information for dosage recommendations for the other drugs.
†Cycle=28 days.
‡The option of splitting the first dose for DARZALEX® is not applicable to DARZALEX FASPRO®.1,2
Fixed-cycle dosing for patients in newly diagnosed, transplant-eligible multiple myeloma1,2*
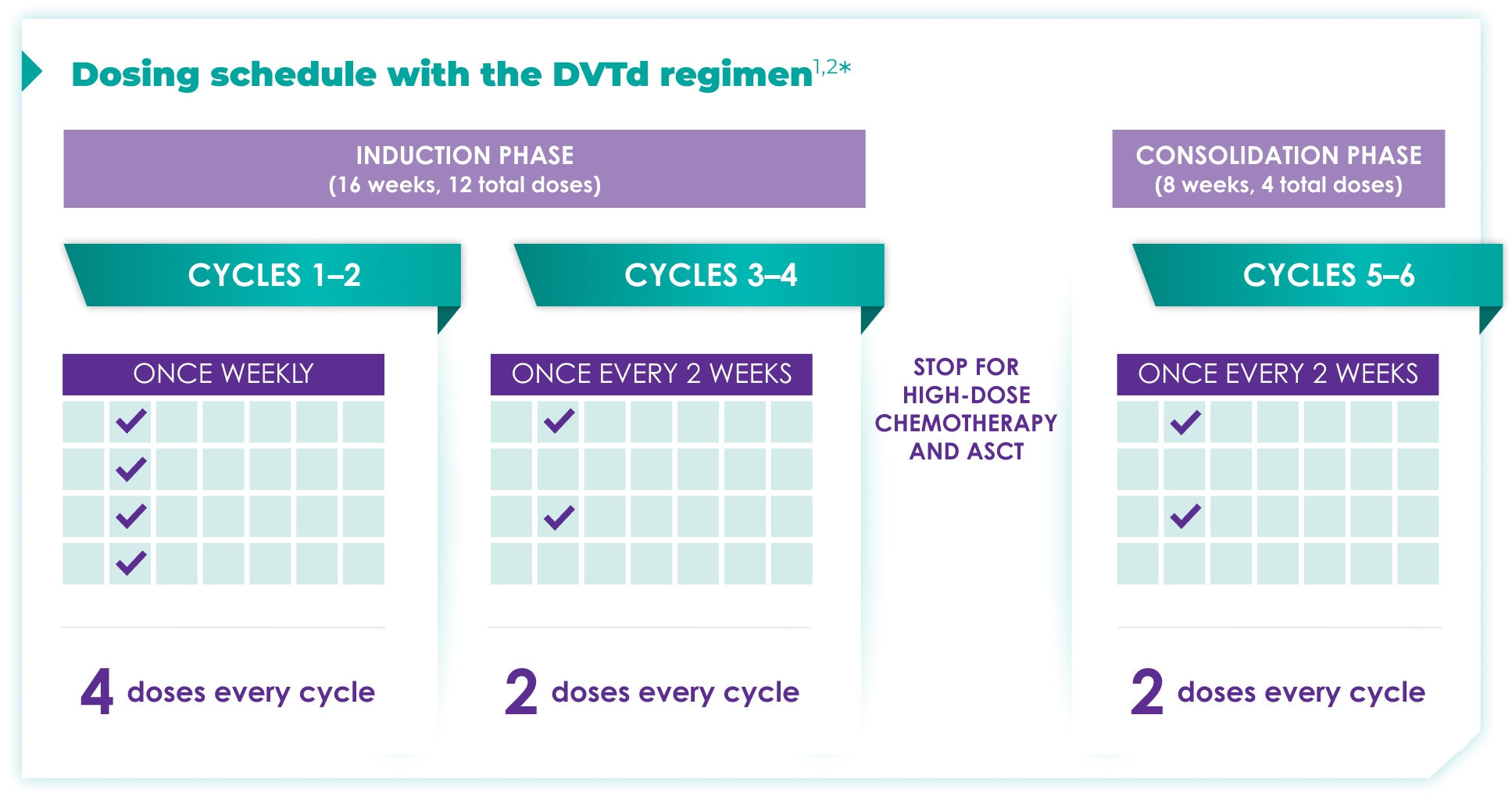
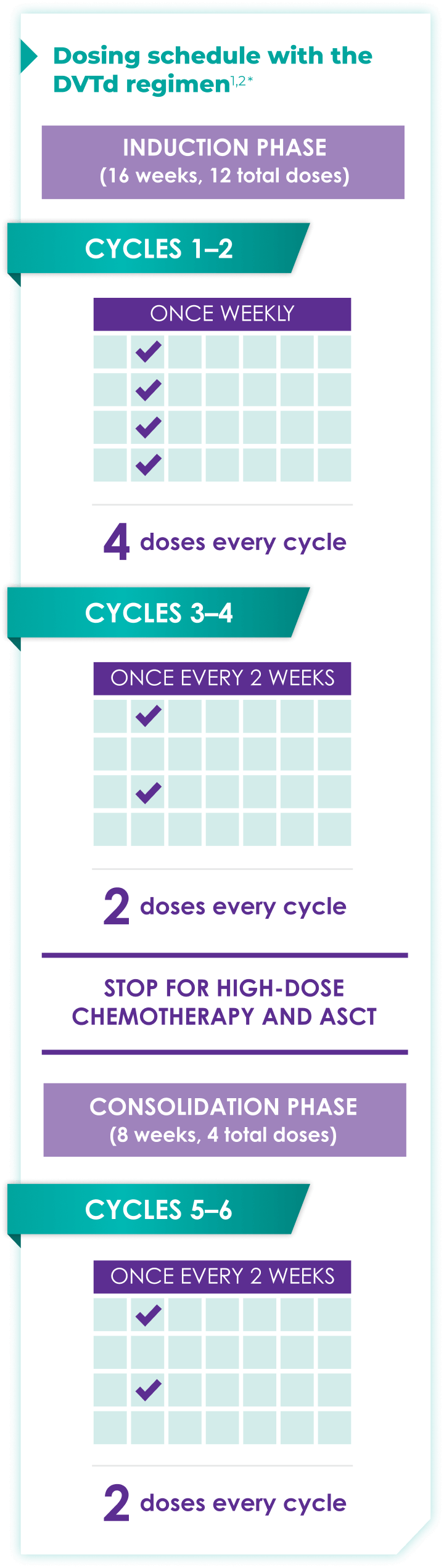
ASCT=autologous stem cell transplant; d=dexamethasone (d); D=DARZALEX FASPRO® (D); DR=DARZALEX FASPRO® (D) + lenalidomide (R); DVRd=DARZALEX FASPRO® (D) + bortezomib (V) + lenalidomide (R) + dexamethasone (d).
DARZALEX FASPRO® in combination with bortezomib + thalidomide + dexamethasone (4-week cycle) has the same dosing schedule as DARZALEX® in combination with bortezomib + thalidomide + dexamethasone.1,2‡
*The first dose of the every-2-week dosing schedule is given at Week 9. First dose of the every-4-week dosing schedule is given at Week 25.1,2
See the Dosage and Administration section of the full Prescribing Information for more details. When DARZALEX FASPRO® or DARZALEX® is administered as part of a combination therapy, see the Prescribing Information for dosage recommendations for the other drugs.
†Cycle=28 days.
‡The option of splitting the first dose for DARZALEX® is not applicable to DARZALEX FASPRO®.1,2
Once-monthly dosing over time—a key milestone for patients to reach in relapsed or refractory multiple myeloma1,2*
Starting at Cycle 7, patients will transition to approximately once-monthly dosing for DARZALEX FASPRO® or DARZALEX®
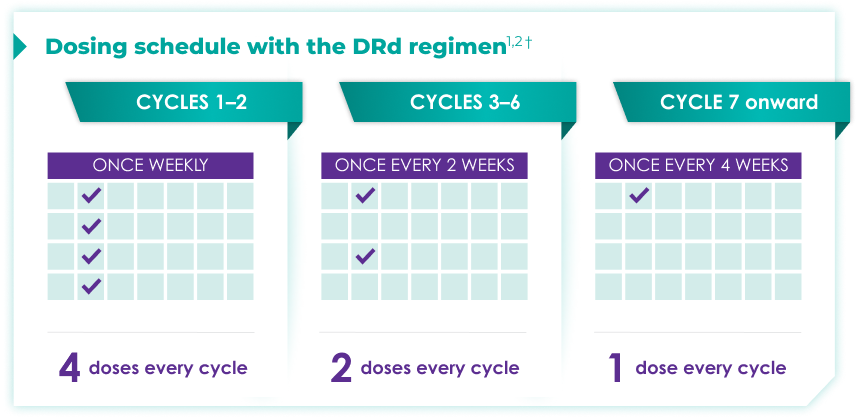
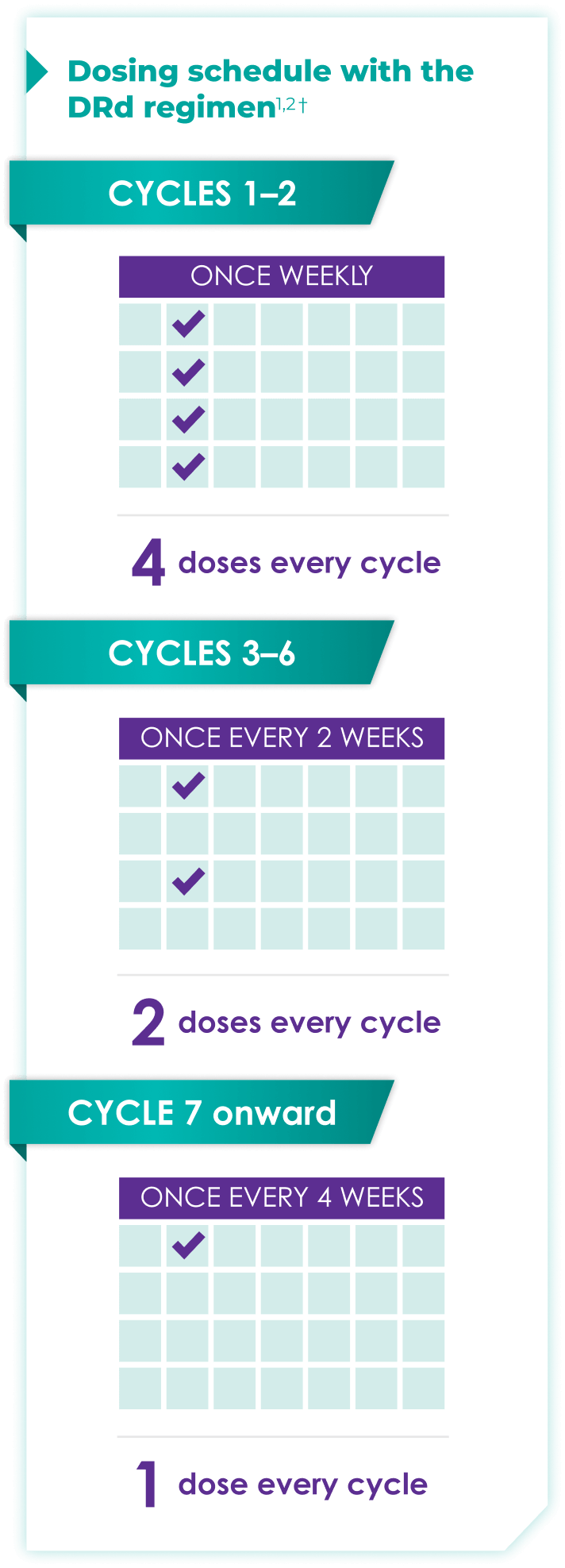
Continue DRd until disease progression or unacceptable toxicity
DRd=DARZALEX FASPRO®/DARZALEX® (D) + lenalidomide (R) + dexamethasone (d).
DARZALEX FASPRO® in combination with lenalidomide and dexamethasone (4-week cycle) has the same dosing schedule as DARZALEX® in combination with lenalidomide and dexamethasone.1,2‡
*The first dose of the every-2-week dosing schedule is given at Week 9. First dose of the every-4-week dosing schedule is given at Week 25.1,2
See the Dosage and Administration section of the full Prescribing Information for more details. When DARZALEX FASPRO® or DARZALEX® is administered as part of a combination therapy, see the Prescribing Information for dosage recommendations for the other drugs.
†Cycle=28 days.
‡The option of splitting the first dose for DARZALEX® is not applicable to DARZALEX FASPRO®.1,2
Once-monthly dosing over time—a key milestone for patients to reach in relapsed or refractory multiple myeloma1,2*
Starting at Cycle 9, patients will transition to approximately once-monthly dosing for DARZALEX FASPRO® or DARZALEX®
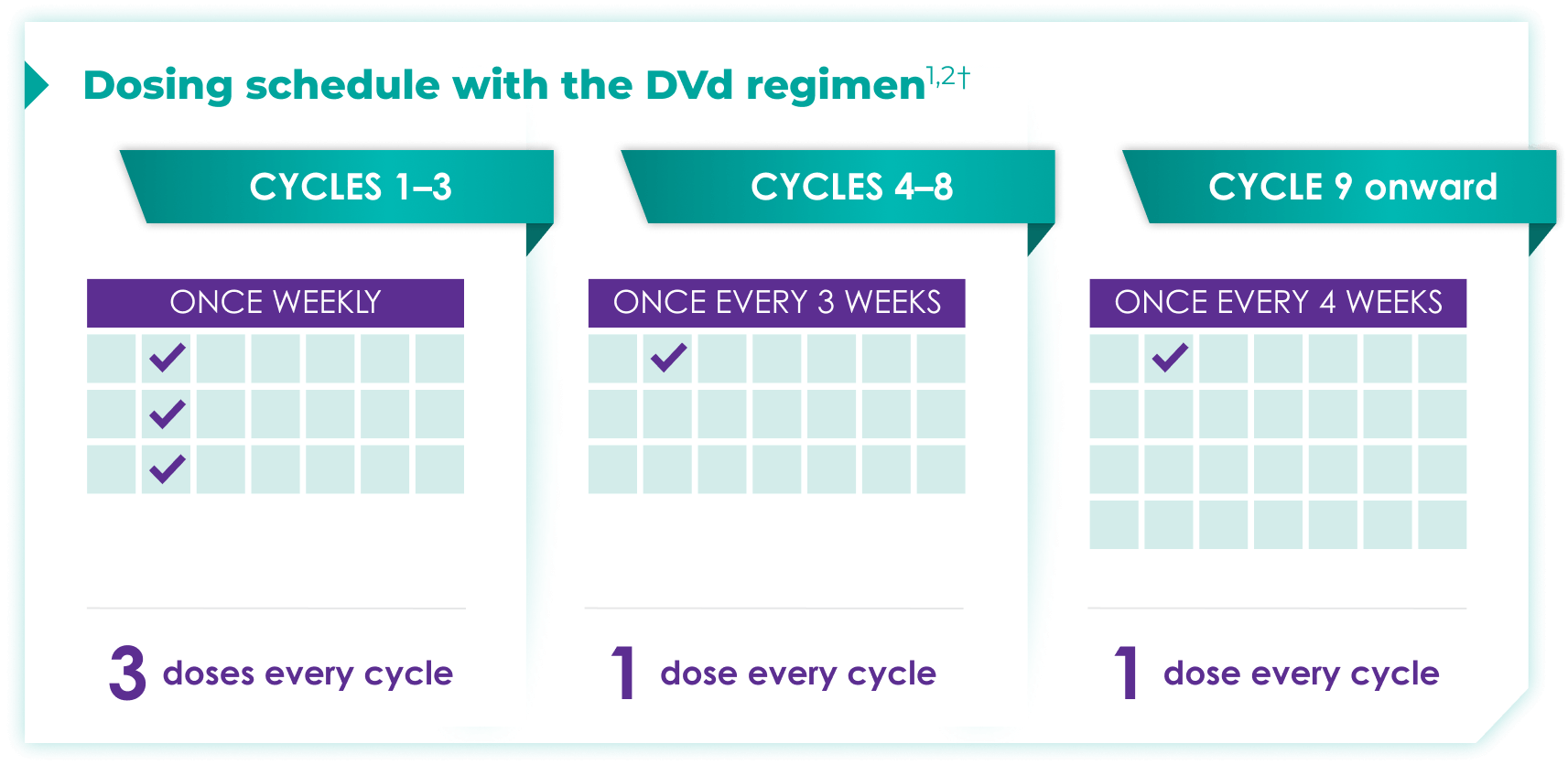
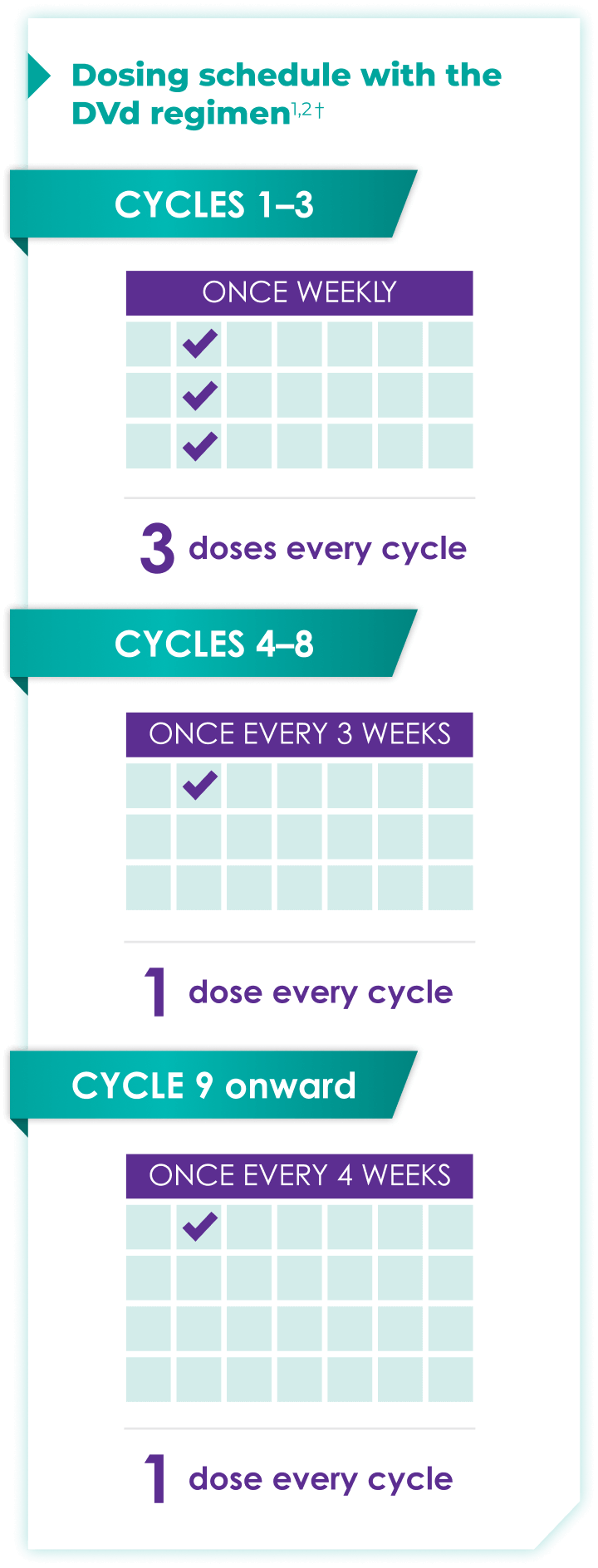
Continue DVd until disease progression or unacceptable toxicity
DVd=DARZALEX FASPRO®/DARZALEX® (D) + bortezomib (V) + dexamethasone (d).
DARZALEX FASPRO® in combination with bortezomib and dexamethasone (3-week cycle) has the same dosing schedule as DARZALEX® in combination with bortezomib and dexamethasone.1,2‡
*The first dose of the every-2-week dosing schedule is given at Week 9. First dose of the every-4-week dosing schedule is given at Week 25.1,2
See the Dosage and Administration section of the full Prescribing Information for more details. When DARZALEX FASPRO® or DARZALEX® is administered as part of a combination therapy, see the Prescribing Information for dosage recommendations for the other drugs.
†Cycle=28 days.
‡The option of splitting the first dose for DARZALEX® is not applicable to DARZALEX FASPRO®.1,2
Once-monthly dosing over time—a key milestone for patients to reach in relapsed or refractory multiple myeloma1,2*
Starting at Cycle 7, patients will transition to approximately once-monthly dosing for DARZALEX FASPRO® or DARZALEX®
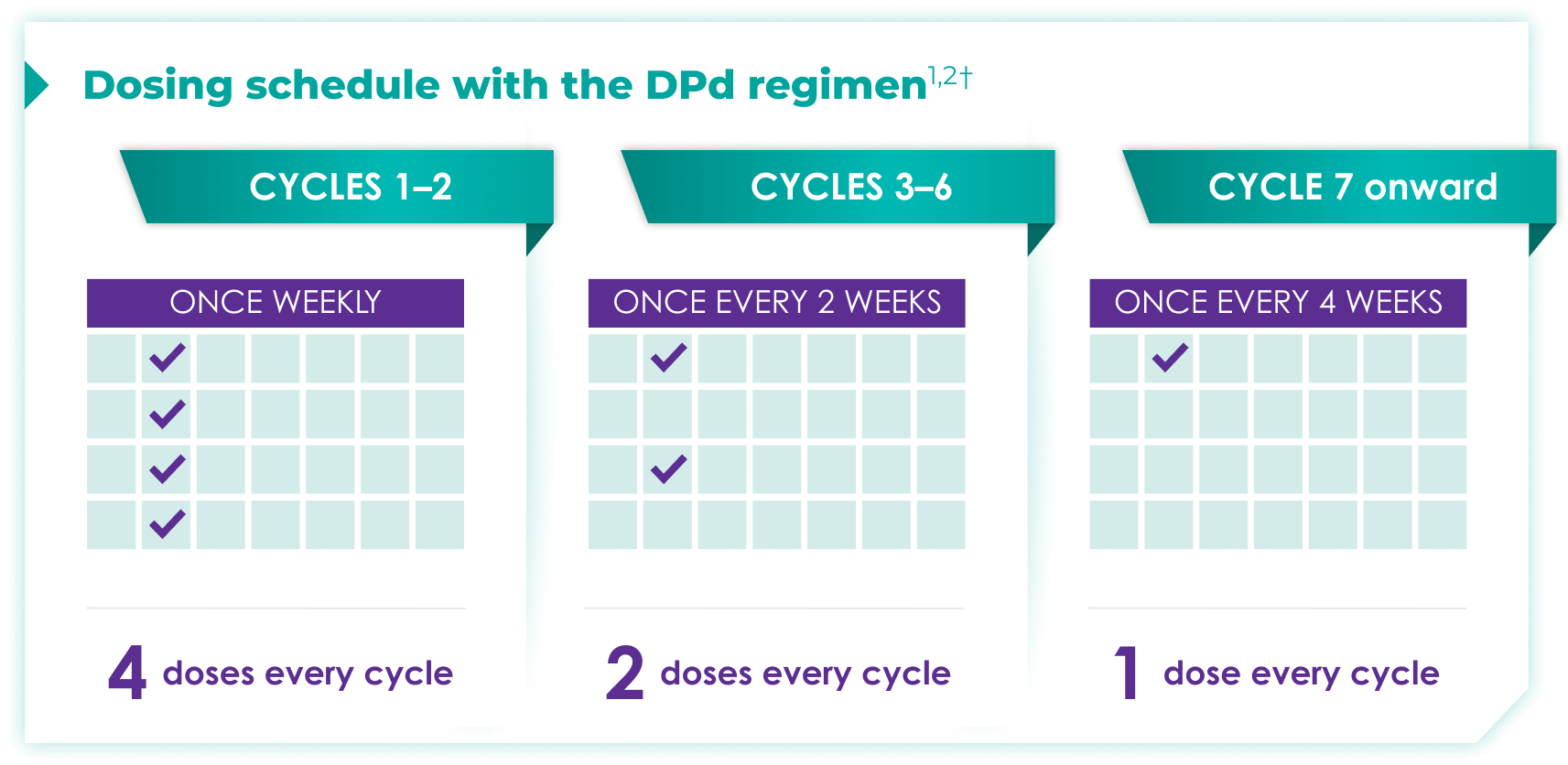
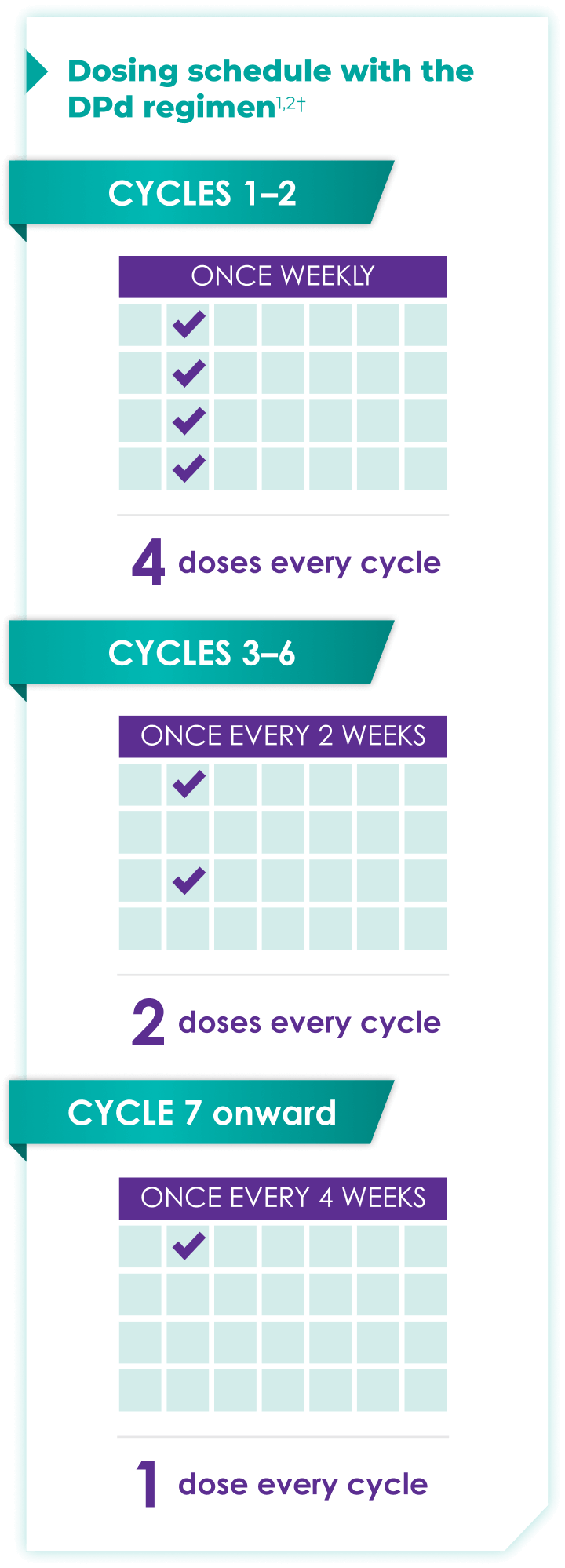
Continue DPd until disease progression or unacceptable toxicity
ASCT:=autologous stem cell transplant; DPd=DARZALEX FASPRO®/DARZALEX® (D) + pomalidomide (P) + dexamethasone (d).
DARZALEX FASPRO® in combination with pomalidomide and dexamethasone (4-week cycle) has the same dosing schedule as DARZALEX® in combination with pomalidomide and dexamethasone.1,2‡
*The first dose of the every-2-week dosing schedule is given at Week 9. First dose of the every-4-week dosing schedule is given at Week 25.1,2
See the Dosage and Administration section of the full Prescribing Information for more details. When DARZALEX FASPRO® or DARZALEX® is administered as part of a combination therapy, see the Prescribing Information for dosage recommendations for the other drugs.
†Cycle=28 days.
‡The option of splitting the first dose for DARZALEX® is not applicable to DARZALEX FASPRO®.1,2
Once-monthly dosing over time—a key milestone for patients to reach in relapsed or refractory multiple myeloma1,2*
Starting at Cycle 7, patients will transition to approximately once-monthly dosing for DARZALEX FASPRO® or DARZALEX®
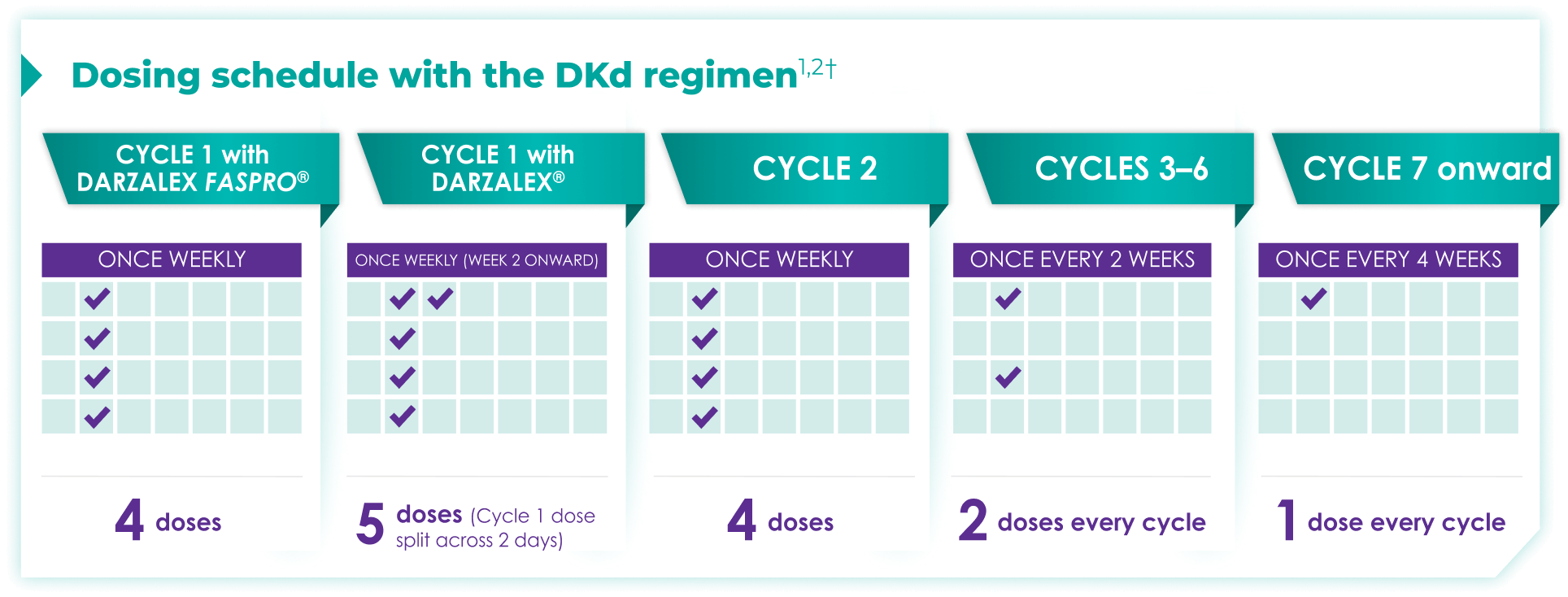
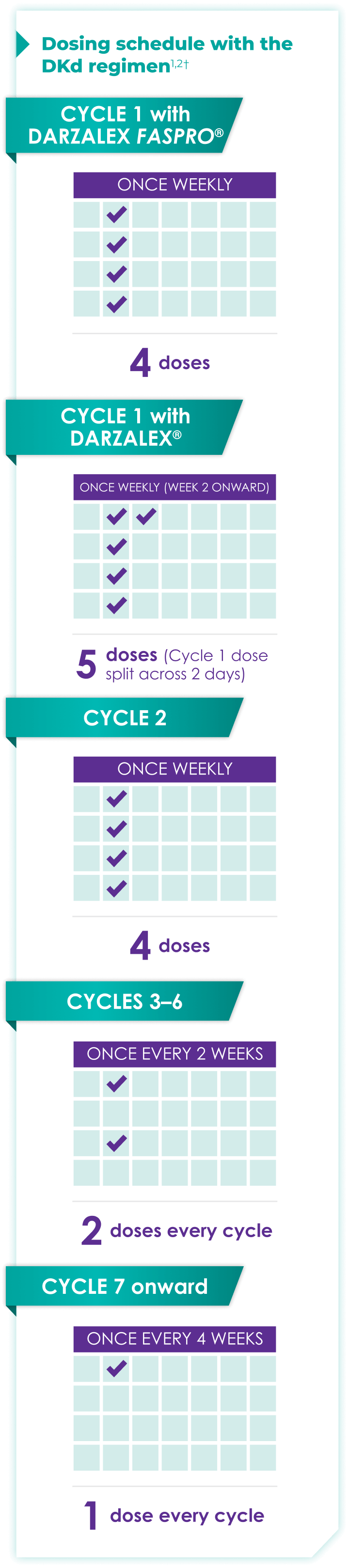
Continue DKd until disease progression or unacceptable toxicity
DKd=DARZALEX FASPRO®/DARZALEX® (D) + carfilzomib (K) + dexamethasone (d).
DARZALEX FASPRO® in combination with carfilzomib and dexamethasone (4-week cycle) has the same dosing schedule as DARZALEX® in combination with carfilzomib and dexamethasone, with the exception of Week 1.1,2
*The first dose of the every-2-week dosing schedule is given at Week 9. First dose of the every-4-week dosing schedule is given at Week 25.1,2
See the Dosage and Administration section of the full Prescribing Information for more details. When DARZALEX FASPRO® or DARZALEX® is administered as part of a combination therapy, see the Prescribing Information for dosage recommendations for the other drugs.
†Cycle=28 days.
‡The option of splitting the first dose for DARZALEX® is not applicable to DARZALEX FASPRO®.1,2
Once-monthly dosing over time—a key milestone for patients to reach in relapsed or refractory multiple myeloma1,2*
Starting at Cycle 7, patients will transition to approximately once-monthly dosing for DARZALEX FASPRO® or DARZALEX®
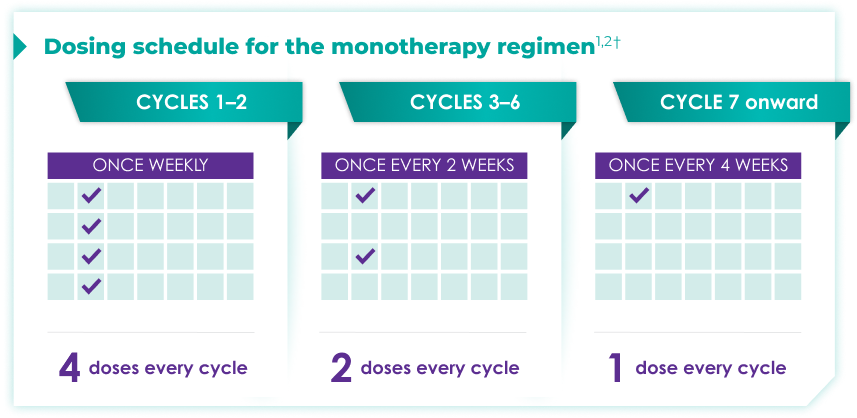
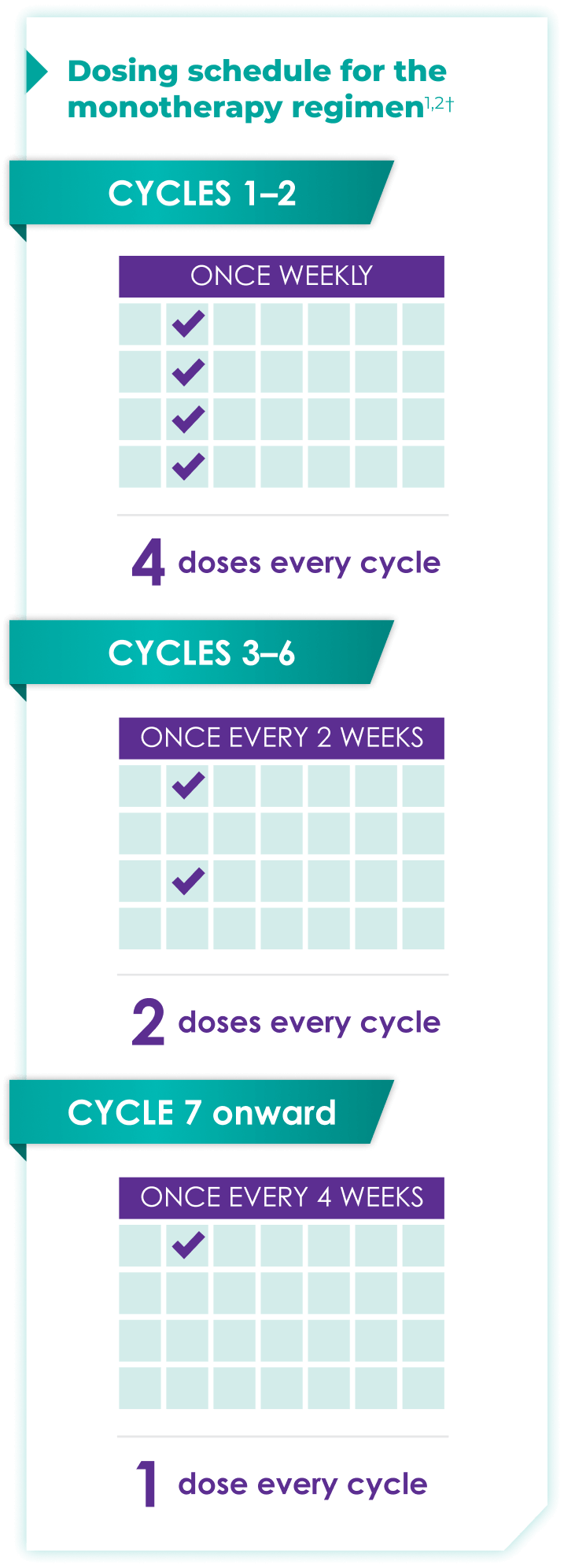
Continue monotherapy treatment until disease progression or unacceptable toxicity
DARZALEX FASPRO® monotherapy (4-week cycle) has the same dosing schedule as DARZALEX® monotherapy.1,2‡
*The first dose of the every-2-week dosing schedule is given at Week 9. First dose of the every-4-week dosing schedule is given at Week 25.1,2
See the Dosage and Administration section of the full Prescribing Information for more details. When DARZALEX FASPRO® or DARZALEX® is administered as part of a combination therapy, see the Prescribing Information for dosage recommendations for the other drugs.
†Cycle=28 days.
‡The option of splitting the first dose for DARZALEX® is not applicable to DARZALEX FASPRO®.1,2
