Hypothetical patient
Philip is a transplant-eligible patient with a moderately active lifestyle
62 years old
Information systems manager
- Married; with 2 adult children
- Enjoys going on long walks with his spouse and pet dog
- Actively manages his health and treatment plan
Newly diagnosed and eligible for a transplant
Transplant eligibility of an individual patient is determined based on evaluation by the treating physician.
Clinical condition and disease presentation
- ISS disease stage: I
- ECOG PS: 1
- Cytogenetic risk: Standard*
Additional considerations
- Mild anemia (9 g/dL)
- Shortness of breath
Presents with:
Discover PERSEUS trial results relevant for treating patients like Philip
THE PERSEUS TRIAL: DARZALEX FASPRO® + VRd
Approval for the treatment of transplant-eligible patients with newly diagnosed multiple myeloma was based on the results of a phase 3 randomized, multicenter, open-label trial1,2
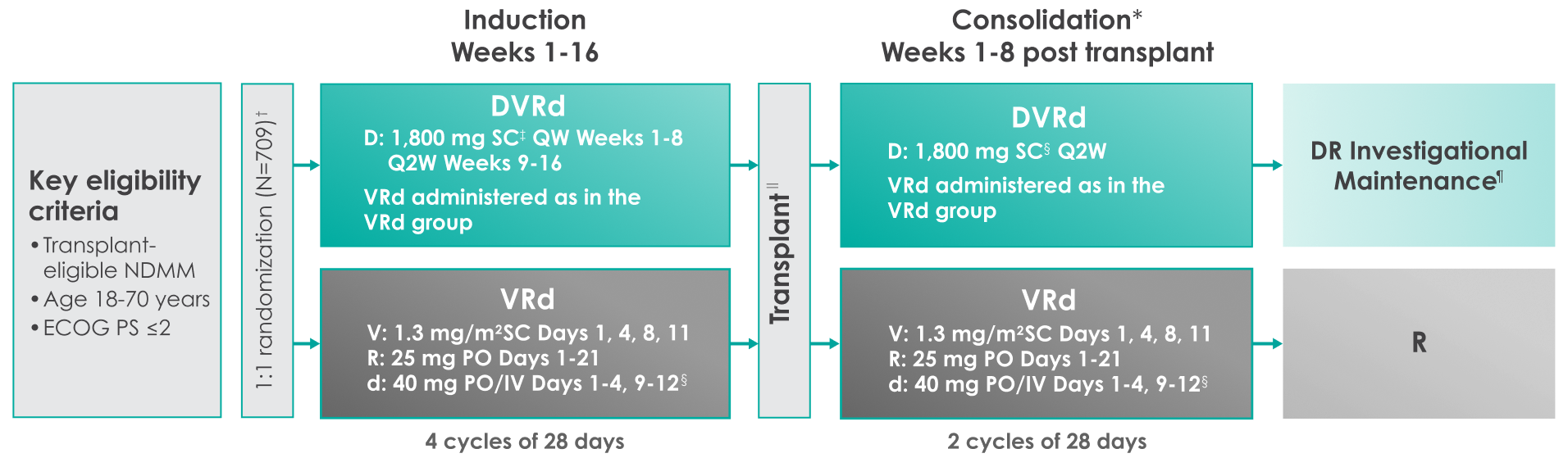
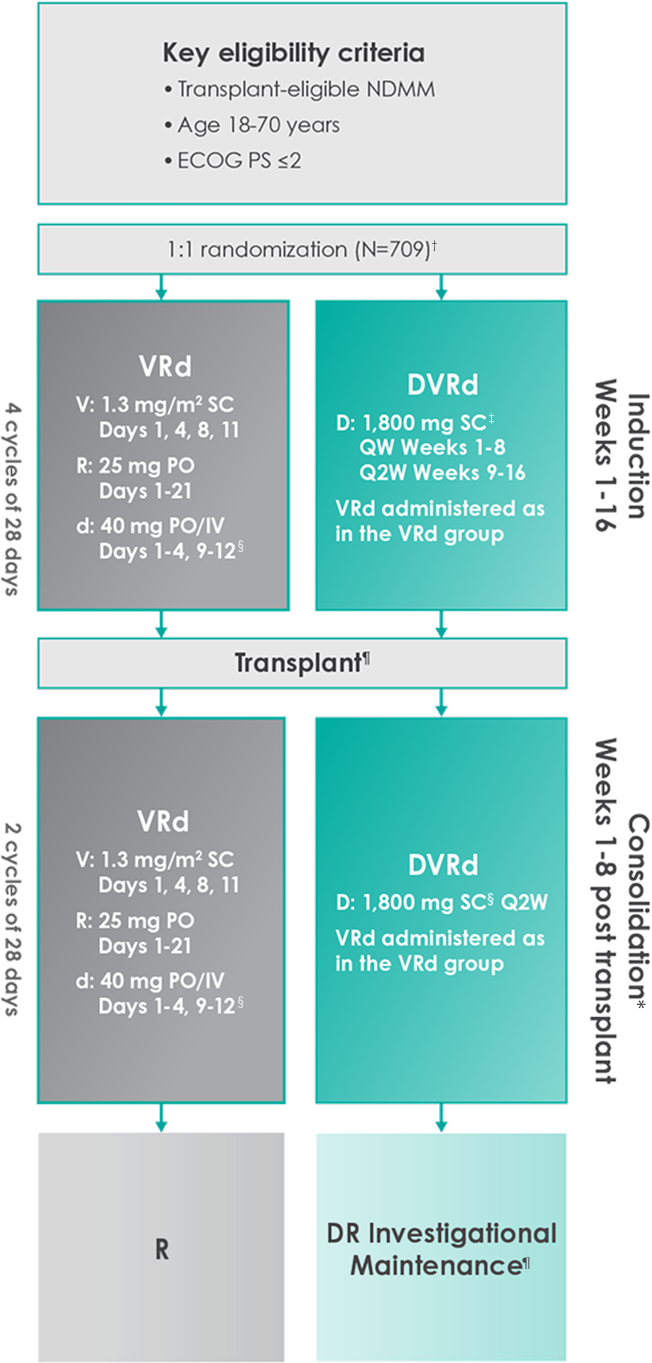
Primary endpoint was progression-free survival (PFS) based on International Myeloma Working Group (IMWG) criteria.1
Key secondary endpoints included overall response rate (ORR), minimal residual disease (MRD) negativity rate (next-generation sequencing [NGS];10-5).1,2#
Is your patient a potential fit for DARZALEX FASPRO® + VRd?
Baseline demographics and disease characteristics were similar between the 2 treatment groups2,3

| Patient Characteristics | DARZALEX FASPRO® + VRd (n=355) | VRd (n=354) |
|---|---|---|
| Median age (y) | 61 | 59 |
| Age category (%) | ||
| <50 yrs | 15 | 15 |
| ≥50 and <65 yrs | 58 | 60 |
| ≥65 yrs | 27 | 25 |
| ECOG PS* (%) | ||
| 0 | 62 | 65 |
| 1 | 32 | 31 |
| 2 | 5 | 5 |
| 3 | 0 | 0 |
| ISS disease stage† (%) | ||
| I | 52 | 50 |
| II | 32 | 35 |
| III | 15 | 14 |
| Cytogenetic profile (%) | ||
| Standard risk | 74 | 75 |
| High risk‡ | 21 | 22 |
| Indeterminate | 4 | 3 |
| Revised standard risk | 49 | 47 |
| Revised high risk§ | 37 | 42 |
| Indeterminate | 14 | 11 |
The PERSEUS trial included patients of various ages, performance status, and cytogenetic abnormalities2
Frontline DARZALEX FASPRO® + VRd significantly reduced the risk of progression or death in patients vs VRd alone after a median follow-up of 47.5 months1,2*
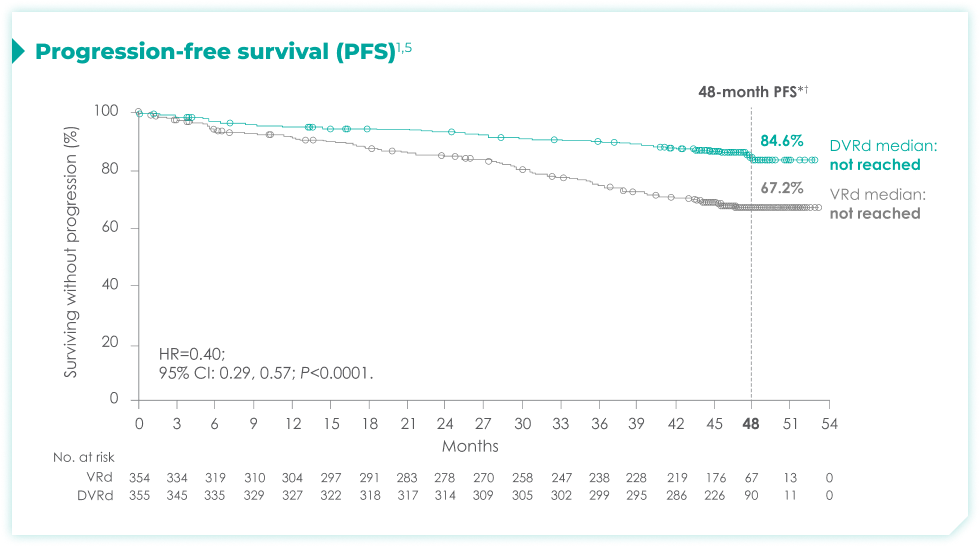
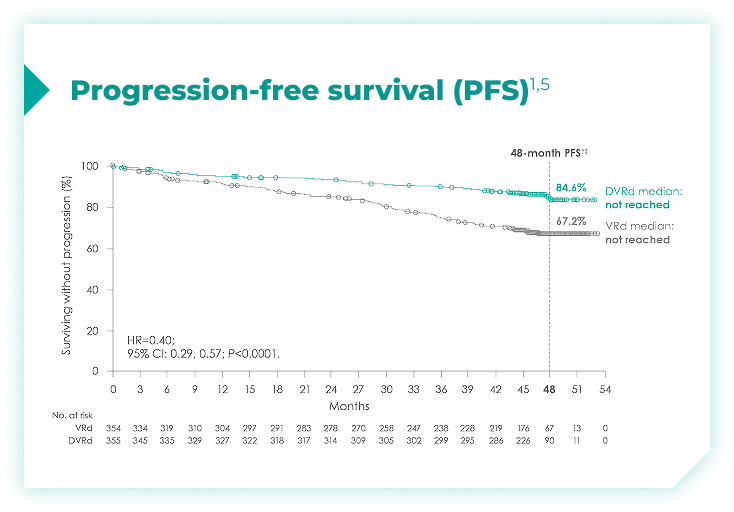
(range: 0.5-18.5 months).1
vs VRd alone (HR=0.40; 95% CI: 0.29-0.57; P<0.0001)1,2*‡
PFS rates in patients with high cytogenetic risk based on the revised definition4*
High cytogenetic risk is defined as having at least 1 out of the following 5 abnormalities: del(17p), t(4;14), t(14;16), gain(1q21), or amp(1q21)4
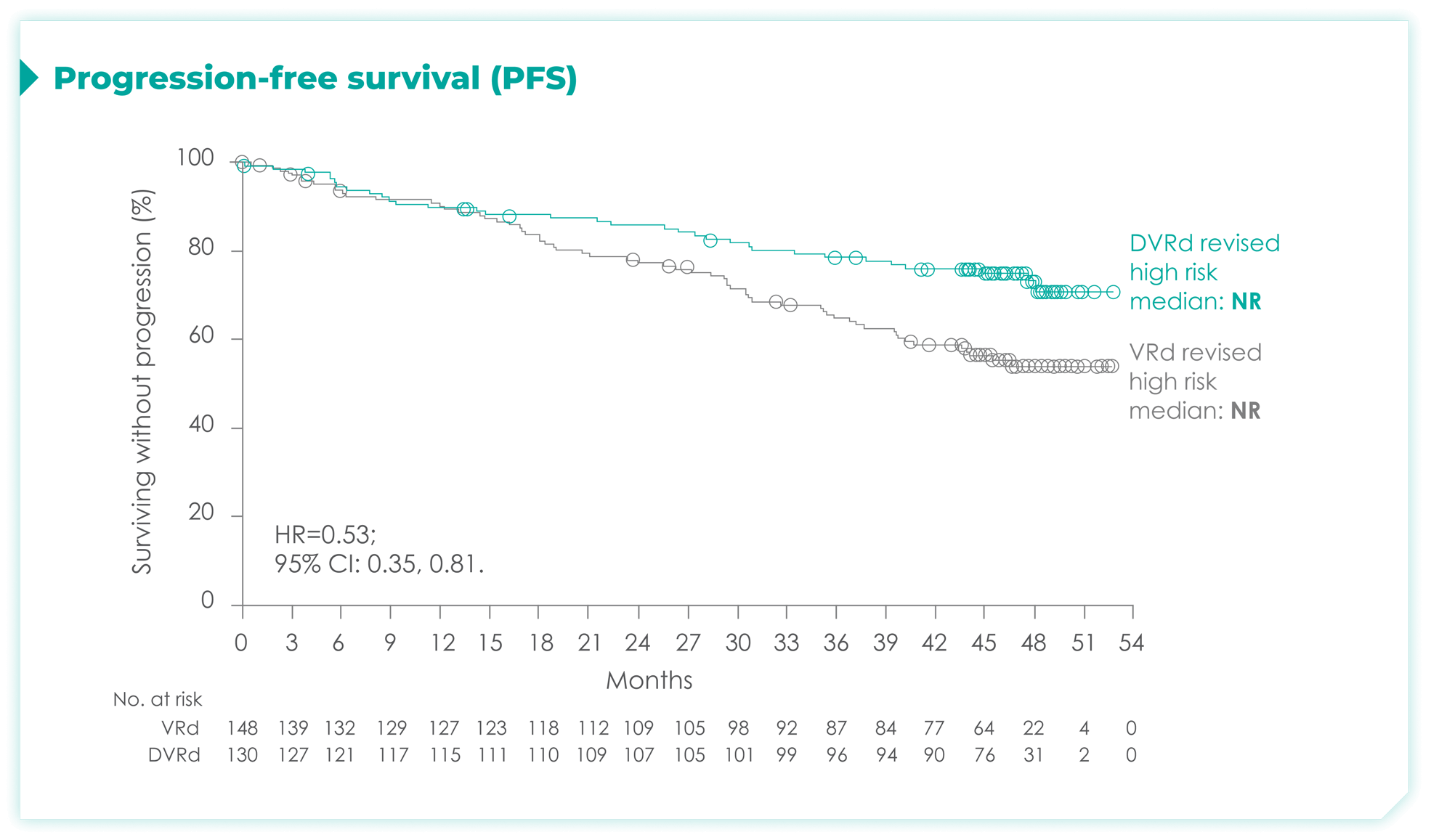
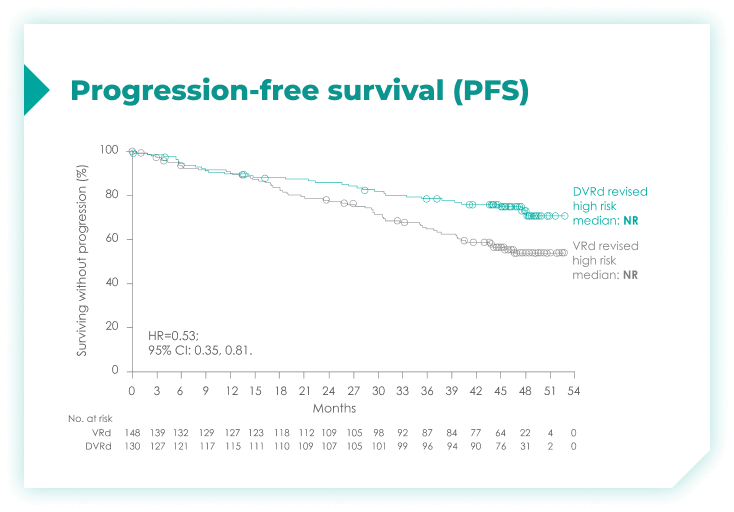
In a subgroup analysis of patients with cytogenetic risk based on the protocol definition: 41% risk reduction for disease progression or death with DARZALEX FASPRO® + VRd vs VRd alone (HR=0.59; 95% CI: 0.39, 0.99)4*
This analysis was conducted post hoc and no conclusions should be drawn.
These results are based on the full treatment regimen and include investigational maintenance of DARZALEX FASPRO® + R following post-transplant consolidation. The trial was not designed to isolate the effect of DARZALEX FASPRO® in the maintenance phase. The efficacy of DARZALEX FASPRO® + R for maintenance has not been established.1
PFS rates in patients based on chromosome 1q21 status4*
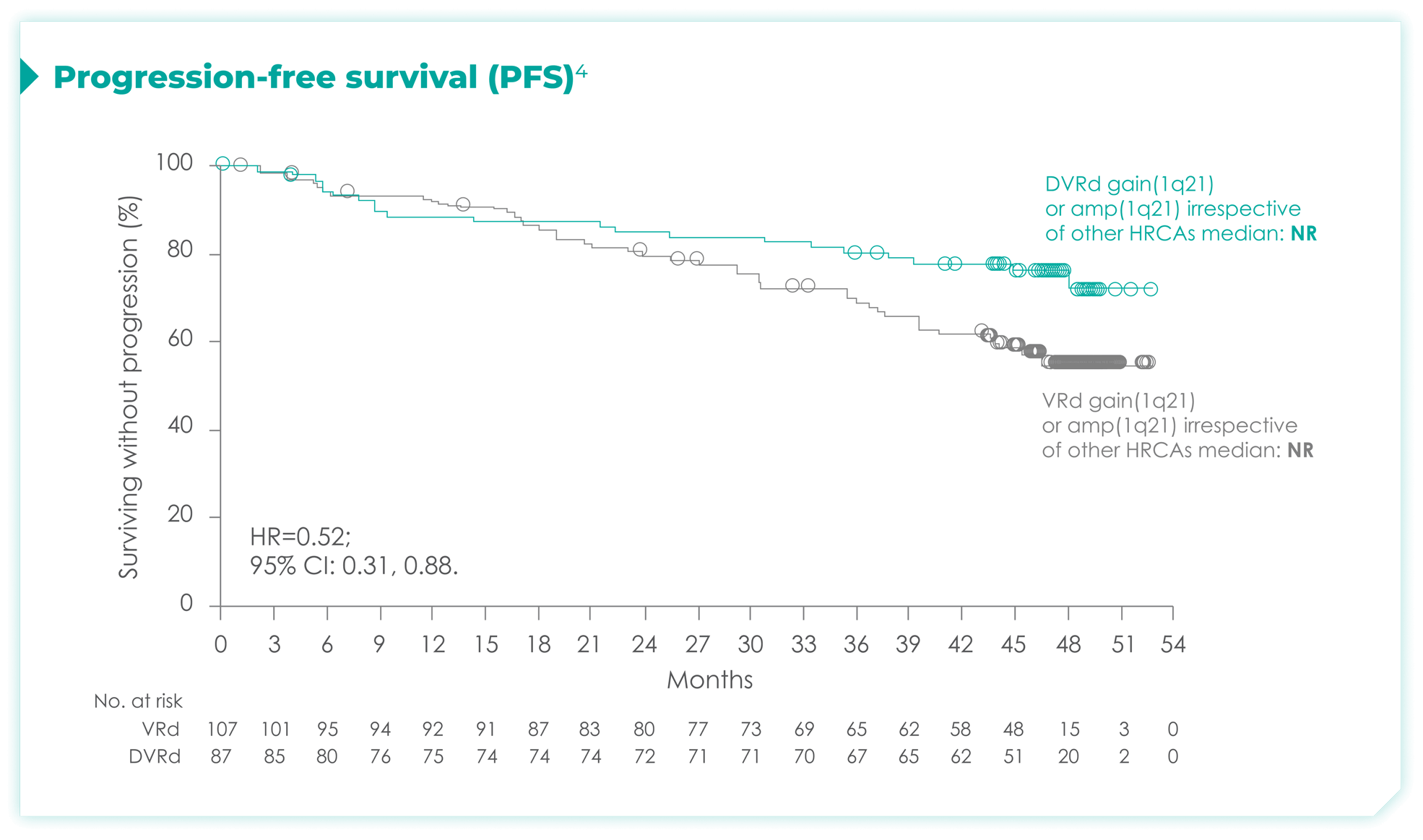
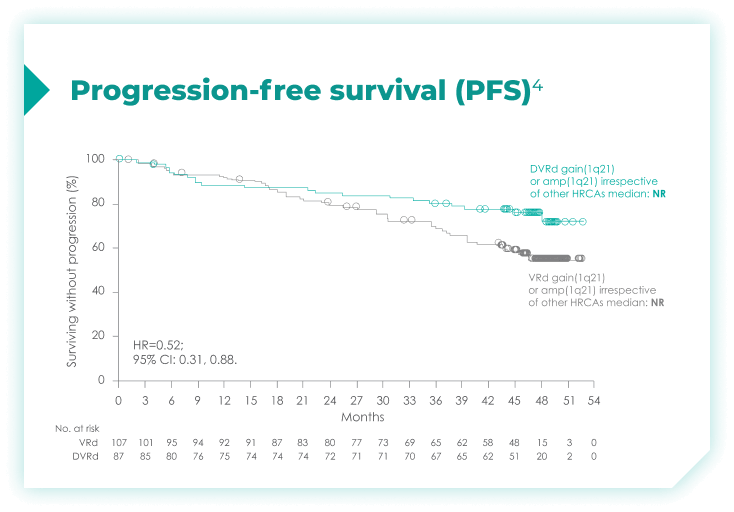
amp=amplification; CI=confidence interval; del=deletion; DVRd=DARZALEX® (D) + bortezomib (V) + lenalidomide (R) + dexamethasone (d); HR=hazard ratio; NR=not reached; PFS=progression-free survival; t=translocation; VRd=bortezomib (V) + lenalidomide (R) + dexamethasone (d).
*Median follow-up was 47.5 months.4
This analysis was conducted post hoc and no conclusions should be drawn.
These results are based on the full treatment regimen and include investigational maintenance of DARZALEX FASPRO® + R following post-transplant consolidation. The trial was not designed to isolate the effect of DARZALEX FASPRO® in the maintenance phase. The efficacy of DARZALEX FASPRO® + R for maintenance has not been established.1
Overall response rates through post-transplant consolidation for frontline DARZALEX FASPRO® + VRd vs VRd alone1*
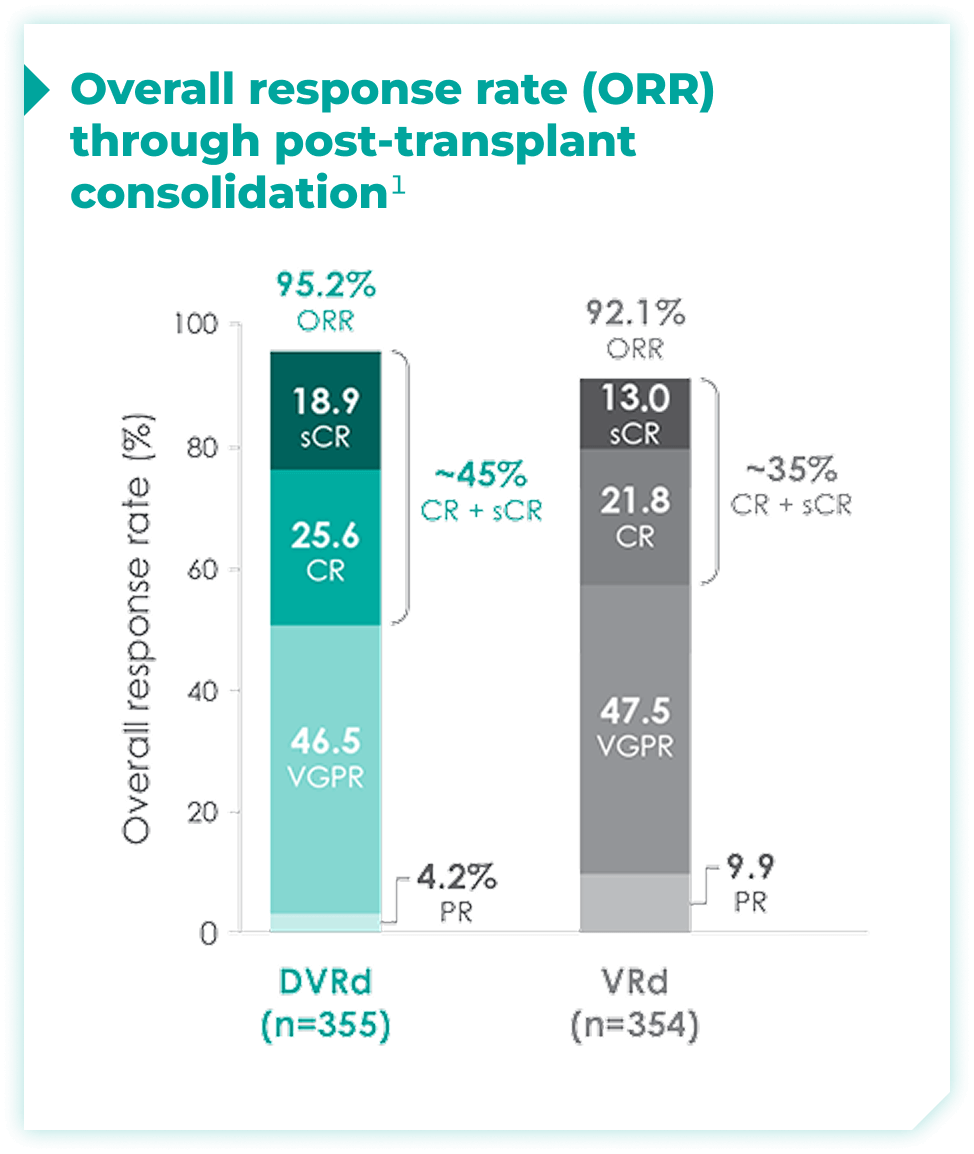
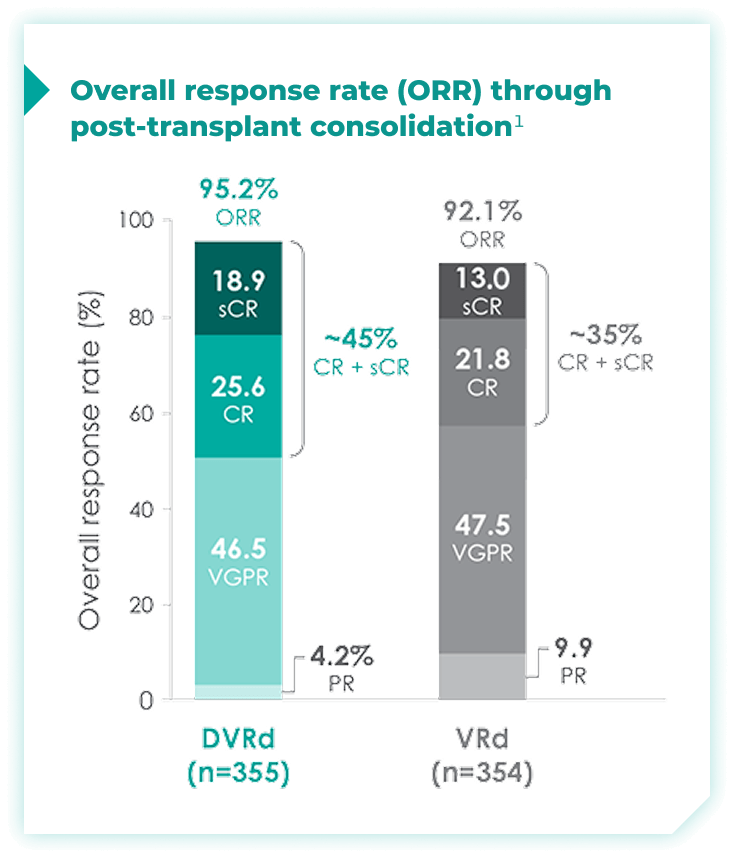
The median time to reach post-transplant consolidation was 9.9 months in the DVRd arm (range: 0.5-18.5 months)1
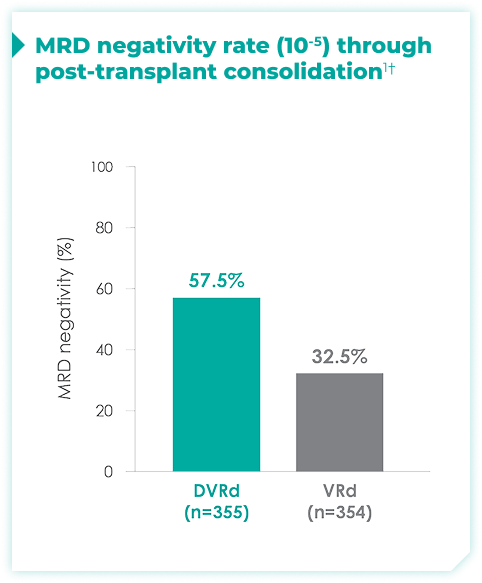
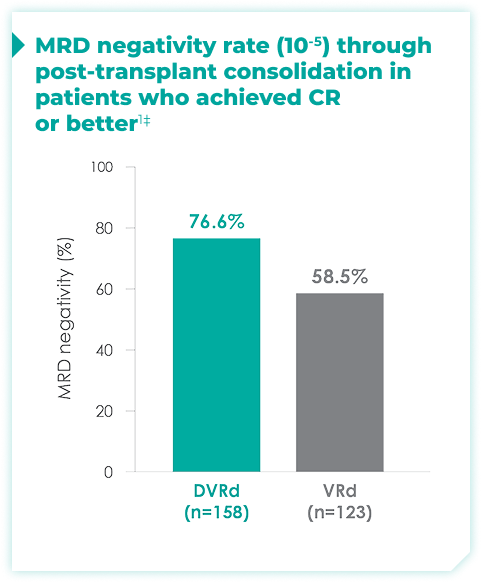
44.5% of patients achieved ≥CR with DVRd, vs 34.7% with VRd alone1
MRD negativity rates through post-transplant consolidation at 10-5 threshold for frontline DARZALEX FASPRO® + VRd vs VRd alone1*
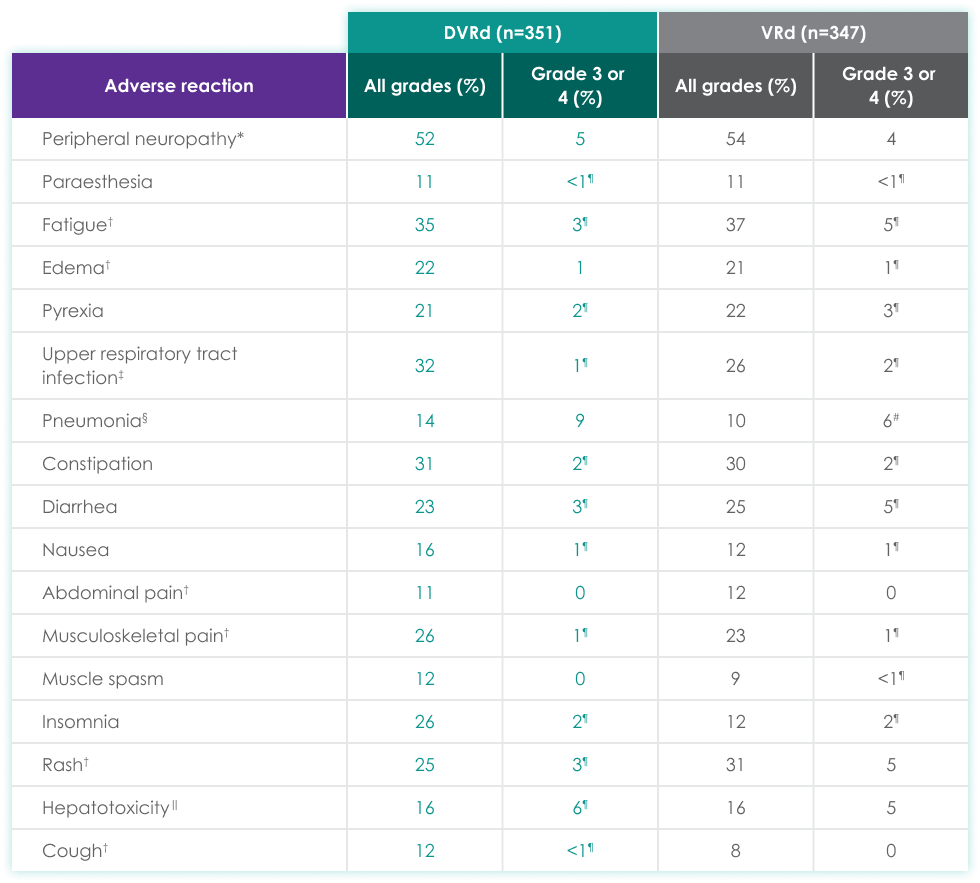
| DVRd (n=351) | VRd (n=347) | |||
|---|---|---|---|---|
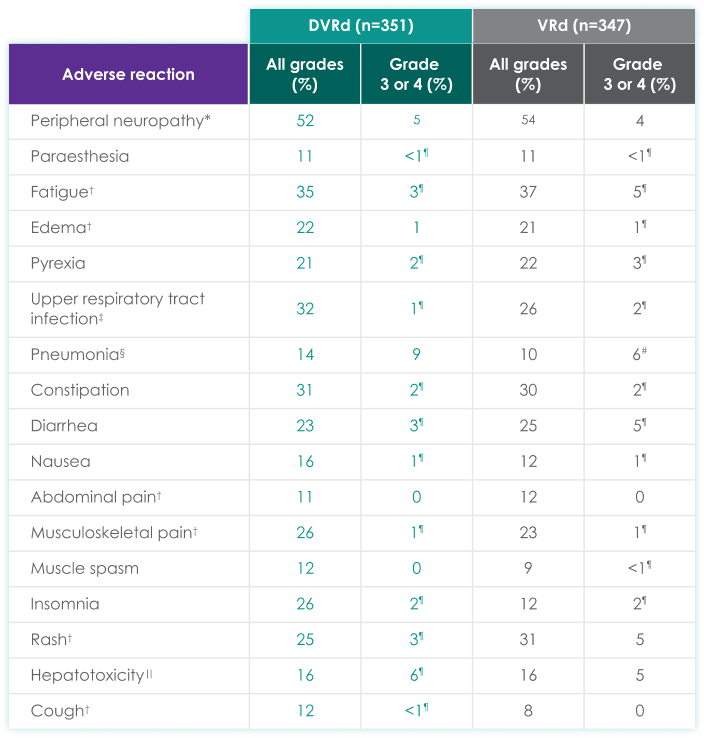
| Adverse reaction | All grades (%) | Grades 3 or 4 (%) | All grades (%) | Grades 3 or 4 (%) |
| Peripheral neuropathy* | 52 | 5 | 54 | 4 |
| Paraesthesia | 11 | <1¶ | 11 | <1¶ |
| Fatigue† | 35 | 3¶ | 37 | 5¶ |
| Edema† | 22 | 1 | 21 | 1¶ |
| Pyrexia | 21 | 2¶ | 22 | 3¶ |
| Upper respiratory tract infection‡ | 32 | 1¶ | 26 | 2¶ |
| Pneumonia§ | 14 | 9 | 10 | 6# |
| Constipation | 31 | 2¶ | 30 | 2¶ |
| Diarrhea | 23 | 3¶ | 25 | 5¶ |
| Nausea | 16 | 1¶ | 12 | 1¶ |
| Abdominal pain† | 11 | 0 | 12 | 0 |
| Musculoskeletal pain† | 26 | 1¶ | 23 | 1¶ |
| Muscle spasm | 12 | 0 | 9 | <1¶ |
| Insomnia | 26 | 2¶ | 12 | 2¶ |
| Rash† | 25 | 3¶ | 31 | 5 |
| Hepatotoxicity‖ | 16 | 6¶ | 16 | 5 |
| Cough† | 12 | <1¶ | 8 | 0 |
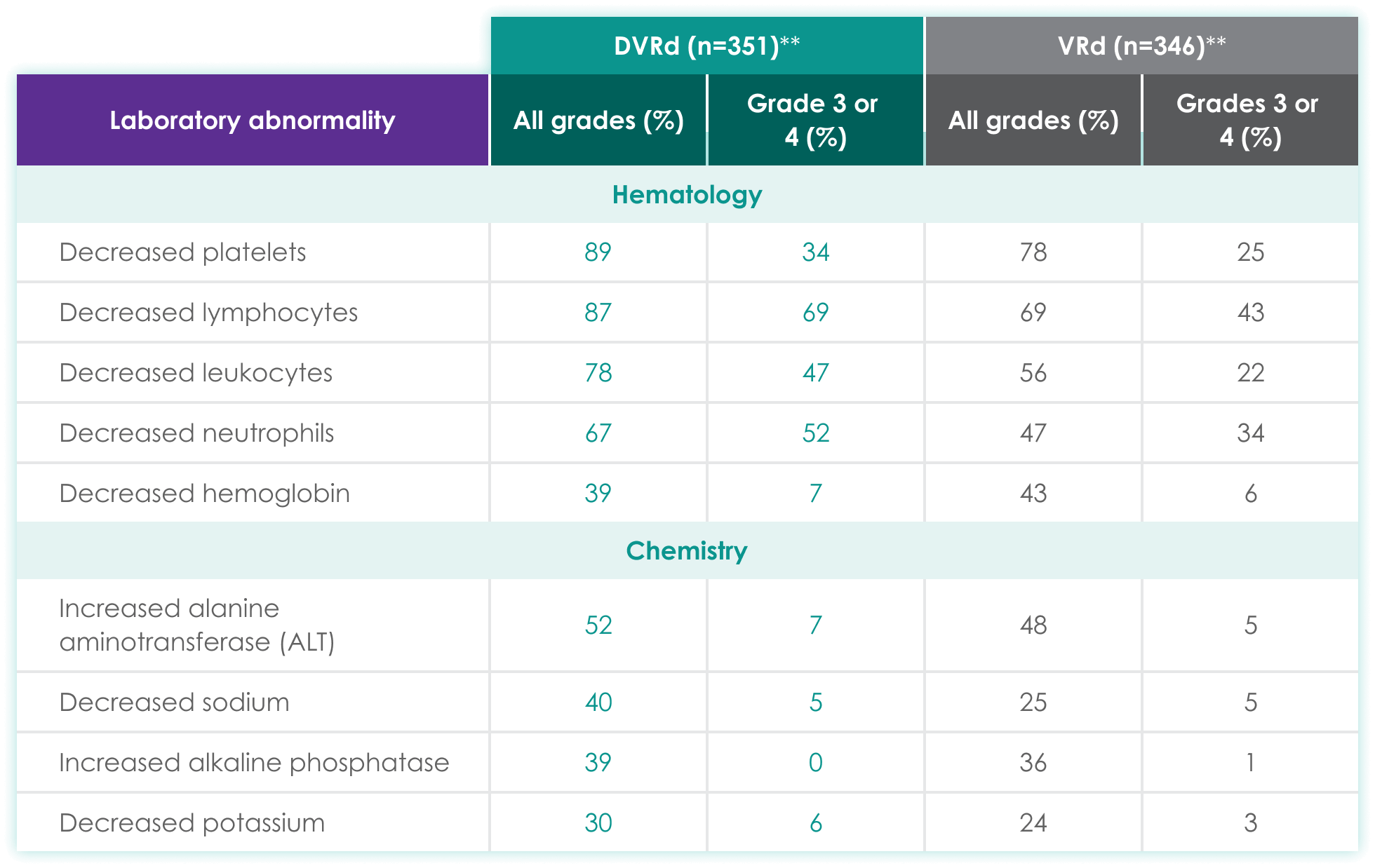
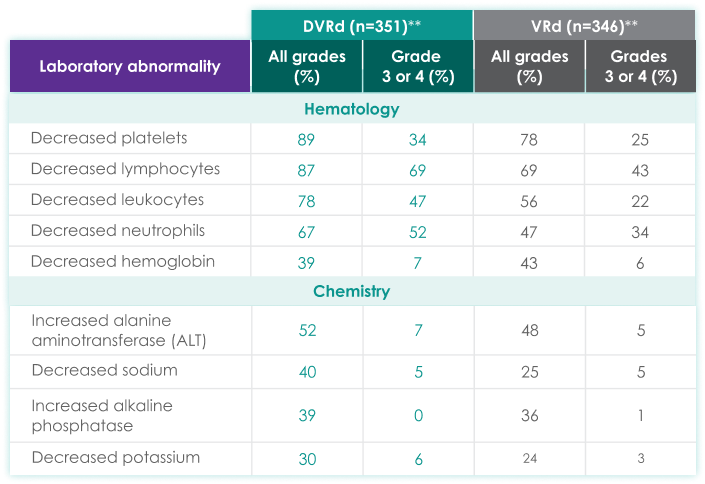
| DVRd (n=351)** | VRd (n=346)** | |||
|---|---|---|---|---|
| Laboratory abnormality | All grades (%) | Grades 3 or 4 (%) | All grades (%) | Grades 3 or 4 (%) |
| Hematology | ||||
| Decreased platelets | 89 | 34 | 78 | 25 |
| Decreased lymphocytes | 87 | 69 | 69 | 43 |
| Decreased leukocytes | 78 | 47 | 56 | 22 |
| Decreased neutrophils | 67 | 52 | 47 | 34 |
| Decreased hemoglobin | 39 | 7 | 43 | 6 |
| Chemistry | ||||
| Increased alanine aminotransferase (ALT) | 52 | 7 | 48 | 5 |
| Decreased sodium | 40 | 5 | 25 | 5 |
| Increased alkaline phosphatase | 39 | 0 | 36 | 1 |
| Decreased potassium | 30 | 6 | 24 | 3 |
- 37% experienced serious adverse reactions
- >5% experienced most frequent serious adverse reactions: pneumonia (6%)
- 1.7% experienced fatal adverse reactions#
- 2% experienced permanent treatment discontinuation due to an adverse reaction
- An adverse reaction which resulted in permanent discontinuation of DVRd in more than 1 patient included sepsis
References:
- DARZALEX FASPRO® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- Sonnenveld P, Dimopoulos MA, Boccadoro M, et al. Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2024;390(4):301-313.
- Sonneveld P, Dimopoulos MA, Boccadoro M, et al. Daratumumab + bortezomib, lenalidomide, and dexamethasone (VRd) versus VRd patients with newly diagnosed multiple myeloma who are eligible for autologous stem cell transplantation: Primary results of the PERSEUS Trial. Oral presentation at: 65th American Society of Hematology (ASH) Annual Meeting; December 9-12, 2023; San Diego, CA.
- Dimopoulos MA, Sonneveld P, Rodriguez-Otero P, et al. Daratumumab (DARA)/bortezomib/
lenalidomide/
dexamethasone (D-VRd) with D-R maintenance in transplant-eligible (TE) newly diagnosed multiple myeloma (NDMM): Analysis of PERSEUS based on cytogenetic risk. Oral presentation at: American Society of Clinical Oncology (ASCO) Annual Meeting; May 31-June 4, 2024; Chicago, IL. - Data on file. Janssen Biotech, Inc.